Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Compounds of Nitrogen. We hope you enjoy the class!
CONTENT
- Oxides of Nitrogen
- Ammonia: Preparation, Properties and Uses.
- Trioxonitrate (V) acid: Preparation, Properties and Uses.
OXIDES OF NITROGEN
NITROGEN (I) OXIDE, N2O
Nitrogen (I) oxide is known as laughing gas as it causes uncontrollable laughter when inhaled.
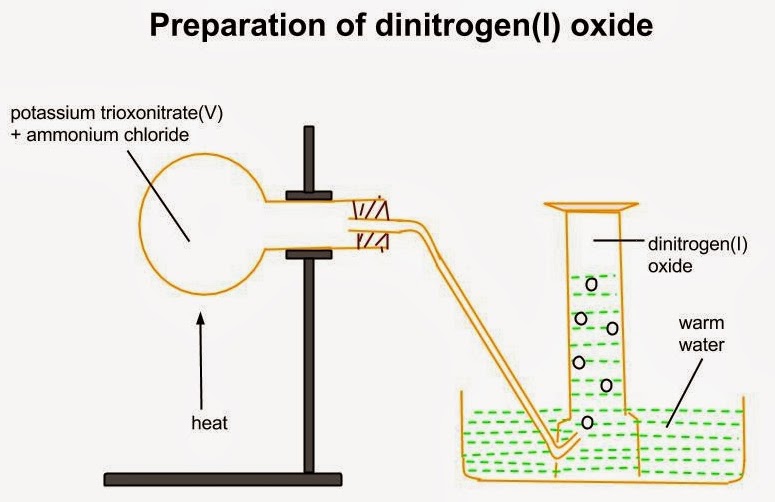
LABORATORY PREPARATION
The gas is prepared in the laboratory by thermal decomposition of ammonium trioxonitrate (V). Ammonium trioxonitrate (V) is not heated directly since the reaction is exothermic and may become uncontrollable leading to an explosion.
a. KNO3(s) + NH4Cl(s) → KCl(s) + NH4NO3(s)
b. NH4NO3(s) → 2H2O(g) + N2O(g)
PHYSICAL PROPERTIES
- It is a colourless gas with a faint pleasant sickly smell and it has a sweetish taste.
- It is fairly soluble in cold water.
- It is 1.5 times denser than air.
- It is neutral to moist litmus paper.
CHEMICAL PROPERTIES
- It decomposes on strong heating (about 600oC) to form nitrogen and oxygen.
2N2O(g) → O2(g) + 2N2(g)
- It supports the combustion of any burning substance which is hot enough to decompose it.
Mg(s) + N2O(g) → MgO(s) + N2(g)
- It is reduced to nitrogen by heated copper or iron
Cu(s) + N2O(g) → N2(g) + CuO(s)
TEST FOR N2O
A glowing splinter is inserted into the gas jar containing the unknown gas. If the splinter is rekindled, the gas is either oxygen or nitrogen (I) oxide. If the gas has a pleasant smell and does not produce brown fumes with nitrogen (IV) oxide; then the gas is nitrogen (I) oxide.
USE: Nitrogen (I) oxide is used as an anesthetic for minor surgical operations.
EVALUATION
- Describe the laboratory preparation of nitrogen (I) oxide.
- Describe a test to distinguish between nitrogen (I) oxide and oxygen gas.
NITROGEN (II) OXIDE, NO
LABORATORY PREPARATION
Nitrogen (II) oxide is prepared by reacting 50% trioxonitrate (IV) acid with copper.
3Cu(s) + 8HNO3(aq) → 3Cu(NO3)2(aq) + 4H2O(l) + 2NO(g)
Some of the nitrogen (II) oxide gas reacts with oxygen in the flask to form brown fumes of nitrogen (IV) oxide which is dissolved in water as the gas is passed through water.
PHYSICAL PROPERTIES
- It is a colourless and poisonous gas.
- It is almost insoluble in water.
- It is slightly denser than air.
- It is neutral to litmus.
CHEMICAL PROPERTIES
- It reacts readily with oxygen to form brown fumes of nitrogen (IV) oxide
2NO(g) + O2(g) → 2NO2(g)
- It decomposes on heating at high temperature to form an equal volume of nitrogen and oxygen
2NO(s)→ N2(g) + O2(g)
- It is reduced to nitrogen by hot metals
2Cu(s) + 2NO(g) → 2CuO(g) + N2(g)
- It acts as reducing agent decolourizing acidified potassium tetraoxomanganate (VI) slowly
3MnO4–(aq) + 4H+(aq) + 5NO(g) → 3Mn2+(aq) + 5NO3–(aq) + 2H2O(l)
TEST FOR NO
- Using air: the gas jar containing the unknown gas is opened, if the gas turns reddish-brown, then the gas is NO.
- Using iron (II) tetraoxosulphate (VI): A solution of FeSO4 which has been acidified with a little dilute H2SO4 acid is poured into the gas jar containing the unknown gas. If the solution turns dark brown, then the gas is NO.
EVALUATION
- Give an equation to show the laboratory preparation of nitrogen (II) oxide.
- State TWO physical and TWO chemical properties of nitrogen (II) oxide.
NITROGEN (IV) OXIDE, NO2
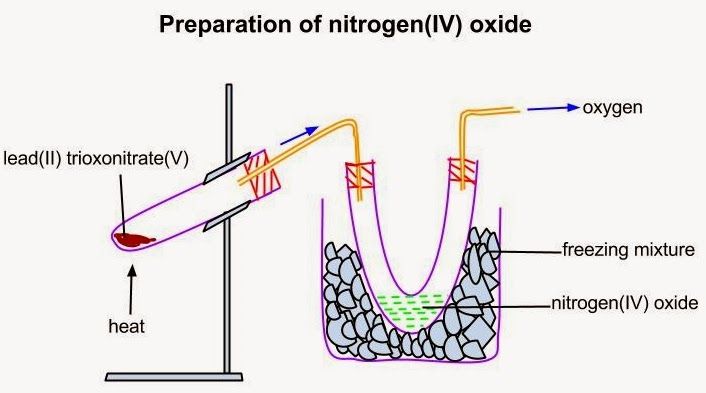
LABORATORY PREPARATION
Nitrogen (IV) oxide is prepared by thermal decomposition of lead (II) trioxonitrate (V) because the nitrate does not contain water of crystallization which can interfere with the preparation.
Pb(NO3)2(s) → 2PbO(s) + O2(g) + 4NO2(g)
The gas mixture obtained is passed through a U- tube immersed in a freezing mixture. Nitrogen (IV) oxide liquefies as a green liquid (yellow if pure) in the tube while oxygen escapes out.
PHYSICAL PROPERTIES
- It is a reddish-brown gas.
- It has an irritating smell and is poisonous.
- It turns damp blue litmus paper red and dissolves in water to form an acidic solution.
- It liquefies into a yellow liquid at 21o
- It is much heavier than air.
CHEMICAL PROPERTIES
- Nitrogen (IV) oxide exists mainly as dinitrogen (IV) oxide, N2O4 at low temperature. It decomposes on heating as follows.
N2O4(g) 2NO2(g) 2NO(g) + O2(g)
Pale Reddish colourless
yellow brown
- It supports combustion as it decomposes on heating to nitrogen and oxygen
2NO2(g) → N2(g) + 2O2(g)
- It is reduced to nitrogen by reducing agent.
4CU(s) + 2NO(g) → 4CuO(s) + N2(g)
- It dissolves in water to form a mixture of dioxonitrate (III) and trioxonitrate (V) acids. It is a mixed acid anhydride.
H2O(l) + 2NO2(g) → HNO2(aq) + HNO3(aq)
- It reacts with alkalis to form mixture of dioxonitrate (III) and trioxonitrate (V) salts
2KOH(aq) + 2NO2(g) → KNO3(aq) + KNO2(aq) + H2O(l)
AMMONIA
Ammonia is a hydride of nitrogen. It is produced in nature when nitrogenous matter decays in the absence of air. Thus, traces of ammonia may be found in the atmosphere but is very soluble in water, it is dissolved by rainwater and washed down into the soil.
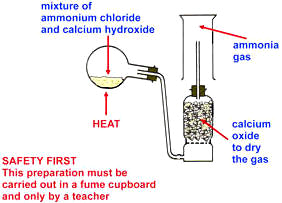
LABORATORY PREPARATION OF AMMONIA
Ammonia is prepared in the laboratory by heating calcium hydroxide, Ca(OH)2 (slaked lime) with ammonium chloride.
Ca(OH)2(s)+ 2NH4Cl(s) → CaCl2(s) +2H2O(l)+2NH3(g).
Ammonia is dried using calcium oxide, CaO. Ammonia being alkaline cannot be dried using Conc. H2SO4 or fused CaCl2, because they will react.
INDUSTRIAL PREPARATION
Ammonia is manufactured from nitrogen and hydrogen by the Haber process. It involves mixing nitrogen and hydrogen in ratio 1:3 by volume. The reaction is reversible so special conditions listed below are required for an optional yield of ammonia.
- Finely divided iron catalyst is used
- A temperature of about 450Oc is used
- A pressure of about 200atm is used.
The yield is about 15% under this condition
N2(g) +3H2(g) 2NH3(g) + heat
PHYSICAL PROPERTIES
- It is a colourless gas with a characteristic choking smell.
- Ammonia in large quantity is poisonous as it affects respiratory muscles.
- It is the only known alkaline gas.
- It is about 1.7 times less dense than air.
- Solid ammonia melts at -34.4OC and the liquid boils at -77.7O
CHEMICAL PROPERTIES
- Ammonia burns readily in oxygen to form water vapor and nitrogen
4NH3(g) + 3O2(g) → 6H2O(g) + 2N2(g)
- Ammonia reacts as reducing agents reacting with
A. Copper II oxide
3CuO(s) + 2NH3(g) → 3Cu(s) + 3H2O(l) + N2(g)
B. Chlorine
3Cl2(g) + 8NH3(g) → 6NH4Cl(s) + N2(g)
3. Ammonia reacts with carbon IV oxide to form Urea and water vapour.
2NH3(g) + CO2(g) → (NH2)2 CO(s) + H2O(l)
urea
4. Ammonia reacts with acid to form ammoniums salts.
2NH3(g) + H2SO4(g) → (NH4)2SO4(s)
TEST FOR AMMONIA
Ammonia has a choking smell. It can be confirmed using:
- Litmus paper: Damped red litmus is dipped into the gas jar containing the unknown gas. If the litmus paper turns blue, then the gas is ammonia.
- Hydrochloric acid: a glass rod is dipped in concentrated HCl and then inserted in the gas jar containing the unknown gas. If white fumes are observed on the glass rod, then the gas is ammonia.
USES OF AMMONIA
- Ammonia is used in the manufacture of trioxonitrate (V) acid and Sodium trioxocarbonate (IV) by the Solvay process.
- Liquid ammonia is used as a refrigerant.
- Aqueous ammonia is used in softening temporary hard water.
- Aqueous ammonia is also used in laundries as a solvent for removing grease and oil stains.
EVALUATION
- Briefly describe the laboratory preparation of ammonia.
- StateTWO physical and THREE chemical properties each of ammonia.
TRIOXONITRATE (V) ACID, HNO3
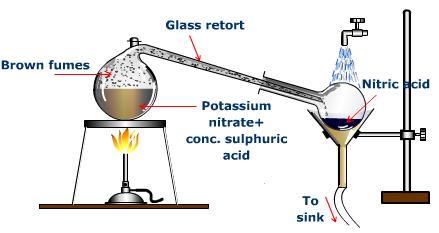
LABORATORY PREPARATION
Trioxonitrate (V) acid is a volatile acid and it is prepared in the laboratory by its displacement from any trioxonitrate salt by concentrated H2SO4 which is less volatile. Trioxonitrate (V) of potassium or sodium is usually used because they are cheap.
KNO3(s) + H2SO4(aq) → KHSO4(aq) + HNO3(aq)
NOTE: An all-glass apparatus must be used in this preparation because the hydrogen trioxonitrate (V) acid vapour will attack cork or rubber.
INDUSTRIAL PREPARATION
Trioxonitrate (V) acid is obtained by the catalytic oxidation of ammonia:
– Ammonia is treated with excess air using Platinum-rhodium catalyst at 700oC to produce nitrogen (II) oxide (96% conversion of NH3 is obtained)
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
– Nitrogen (II) oxide formed is cooled and mixed with excess air to produce nitrogen (IV) oxide.
2NO(g) + O2(g) → 2NO2(g)
– Nitrogen (IV) oxide formed is dissolved with excess air in hot water to yield trioxonitrate (V) acid solution of up to 50% concentration.
4NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
PHYSICAL PROPERTIES
- The pure acid is a colourless fuming liquid with a sharp choking smell. The acid turns yellow due to its decomposition to nitrogen (IV) oxide which redissolves in the acid.
- The pure acid boils at 860C and melts at -47oC
- The density of the pure acid is 1.52 gcm-3
- The pure acid is miscible with water in all properties and forms a constant boiling mixture with it at 121oC
- The concentrated form of the acid is corrosive.
- The dilute acid turns blue litmus red.
CHEMICAL PROPERTIES
- As an acid it neutralizes bases and alkalis to form metallic trioxonitrate (V) and water only
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l)
- As an acids it reacts with metallic trioxocarbonate (IV) to liberate carbon (II) oxide
CaCO3(s) + HNO3(aq) → Ca(NO3)2(aq) + H2O(l) + CO2(g)
- Unlike other acids, it rarely gives out hydrogen with metals except when very dilute solution is reacted with Ca, Mg or Mn.
- As an oxidizing agent, it reacts with non – metal to form the corresponding oxides of the non – metals.
S(s) + 6HNO3(aq) → H2SO4(aq) + 2H2O(l) + 6NO2(g)
- As an oxidizing agent, it oxidizes Cu, Pb, Hg and Ag to yield the respective trioxonitrate (V) and nitrogen (IV) oxide if concentrated, but nitrogen (II) oxide if the concentration is moderate.
Aluminum and iron are not oxidized to their oxides by concentrated HNO3(aq) due to formation of a surface coating of oxide which is passive do not allow further reaction with the metals. Aluminum or iron lined container can be used to transport concentrated HNO3(aq)
- As an oxidizing agent, it oxidizes hydrogen sulphide to sulphur
H2S(g) + 2HNO3(aq) → S(s) + 2H2O(l) + 2NO2(g)
- As an oxidizing agent, it oxidizes iron (II) salts to iron (III) salts
6Fe2+(aq) + 8H+(aq) + 2NO3–(aq) → 6Fe3+(aq) + 4H2O(l) + 2NO(g)
USES
- It is used as an acid, oxidizing agent and nitrating agent in the laboratory.
- It is used in nylon production and Terylene.
- It is used as rocket fuel.
- It is used in the production of fertilizers, dyes, drugs and explosives.
GENERAL EVALUATION/REVISION
- Describe the laboratory preparation of trioxonitrate (V) acid.
- Write TWO equations of reactions in which trioxonitrate (V) is acting as an acid.
- Write an equation to show the reaction of nitrogen (IV) oxide as a mixed anhydride.
- Describe the electrolysis of CuSO4 solution using platinum electrodes.
- Classify the following oxides: CuO, Na2O, PbO, NO2, N2O
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Sulphur. We are very much eager to meet you there.
