Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m glad you’re here again. Let me paint a simple picture. Imagine two families living in Lagos—one in a large compound house with plenty of space, and the other in a small self-contained flat. The size of the house affects how much space the family has to spread out. In the same way, atoms and ions also have “sizes,” which we measure using atomic radius and ionic radius.
Atomic And Ionic Radii
What is Atomic Radius?
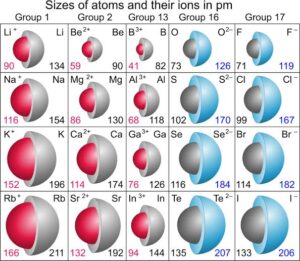
The atomic radius is the distance from the centre of the nucleus of an atom to the outermost shell of electrons. Think of it as how “big” an atom is.

For example:
Sodium atom has an atomic radius of about 186 pm (picometres).
Fluorine atom has an atomic radius of about 64 pm.
Trends in Atomic Radius
Across a period (left to right): Atomic radius decreases. Why? As protons increase, the nucleus pulls electrons closer, shrinking the atom. Example: lithium (larger) vs fluorine (smaller).
Down a group (top to bottom): Atomic radius increases. Why? More electron shells are added, making atoms larger. Example: lithium (smaller) vs caesium (larger).
What is Ionic Radius?
The ionic radius is the size of an ion after an atom either loses or gains electrons.
Cations (positive ions): Formed when atoms lose electrons. They are smaller than the original atom because losing electrons reduces repulsion and the nucleus pulls the remaining electrons closer. Example: Na⁺ is smaller than Na.
Anions (negative ions): Formed when atoms gain electrons. They are larger than the original atom because extra electrons increase repulsion, making the ion expand. Example: Cl⁻ is larger than Cl.
Trends in Ionic Radius
Within the same group of ions, ionic size increases down the group as electron shells increase.
For isoelectronic species (ions with the same number of electrons), the one with more protons is smaller because the nucleus pulls the electrons more strongly. Example: O²⁻ > F⁻ > Na⁺ > Mg²⁺ (all have 10 electrons).
Everyday Examples
Table salt (NaCl): Sodium loses an electron to form Na⁺ (small), while chlorine gains an electron to form Cl⁻ (big). Together, their different sizes influence how they pack into the crystal lattice.
Fertilisers often contain ionic compounds, and their stability depends on the sizes of the ions.
Summary
- Atomic radius: size of an atom from nucleus to outer shell.
- Across a period: decreases; down a group: increases.
- Ionic radius: size of ions; cations are smaller, anions are larger.
- Isoelectronic species: more protons = smaller ion.
Evaluation
- Define atomic radius.
- Explain why Na⁺ is smaller than Na.
- Arrange these ions in order of increasing size: Mg²⁺, Na⁺, O²⁻, F⁻.
Excellent! You’ve now understood how atoms and ions change size, just like houses differ in space. With Afrilearn, Chemistry concepts that once seemed abstract are now clear and relatable. Keep learning—you’re building a strong foundation in science!
