Back to: CHEMISTRY SS1
Welcome to class!
In today’s class, we will be talking about compounds and mixtures. Enjoy the class!
COMPOUNDS AND MIXTURES

COMPOUND
A Compound is a substance which contains two or more elements chemically combined together. A compound is formed as a result of chemical change.
Examples of compounds are
Compound Constituent Elements
- Water Hydrogen, Oxygen
- Sand Silicon, Oxygen
- Limestone calcium, carbon, Oxygen
- Common salt Sodium, Chlorine
- Ethanol Carbon, Hydrogen, Oxygen
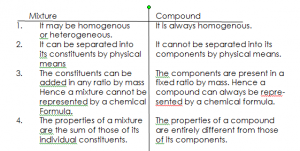
PROPERTIES OF A COMPOUND
- It has properties different from those of its component elements.
- Its formation often requires a large amount of heat.
- It cannot be separated by physical means.
- The components of a compound have a fixed ratio by mass.
- Compounds are homogenous.
EVALUATION
- Define a compound.
- Give three examples of a compound.
FORMULAE OF COMPOUNDS
When an element exists as a molecule, a number is written as a subscript after the symbol of that element. For example, hydrogen is written as H2 and oxygen as 02.
A compound contains whole numbers of atoms of the component elements. Its molecular formula is written as follows.
- The symbols of all the component elements are written close together as a group.
- The number of atoms of each component element is written as a subscript after the symbol of that element.
Examples:
Compound Formula
- Hydrochloric acid HCl
- Water H20
- Ammonia NH3
- Carbon(IV)oxide C02
- Lead II chloride PbCl2
- Calcium trioxonitrate(V) Ca(NO3)2
WRITING FORMULA FROM VALENCIES
Formulae of compounds can be deduced from the valencies of the component elements or radicals, following the rules below.
i. Write the symbols of the element or radicals in a compound
ii. Write their valencies below the symbols of elements/radicals
iii. Exchange their valencies.
iv. Now write the formula of the compound bringing the symbols of the element or radicals together
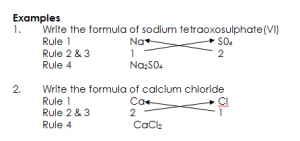
EVALUATION
- Write the formulae of; (i) tetraoxosulphate(vi)acid (ii) Magnesium Chloride
- State three properties of a compound
MIXTURES
A mixture contains two or more constituents which can easily be separated by physical methods.
Examples of mixtures with their constituents are outlined below:
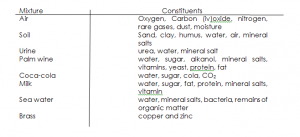
DIFFERENCES BETWEEN MIXTURES AND COMPOUNDS
EVALUATION
- List five (5) compounds and their formulae
- What is a mixture?
- State four differences between compound and mixture
READING ASSIGNMENT
- New School Chemistry for SSS by O.Y Ababio. Pg 11, 36 to 37
GENERAL EVALUATION/REVISION
- State the valency of the following elements and radicals: Na, K, S, O, SO42-, NO3–, CO32-
- Write the formula of a) Lead (ii) tetraoxosulphate (vi) b) Hydrochloric acid c) Sodium trioxocarbonate (iv) d) Calcium hydroxide
WEEKEND ASSIGNMENT
- Which of the following is a mixture? (a) water(b) sugar(c) milk (d) starch
- Which of the following is a compound? (a) water (b) soil (c) diamond (d) graphite
- Which of these formulae represents ammonia? (a) NH3 (b) NH4+ (c) NH2 (d) CH4
- The formula for sand is (a) C02 (b) SO2 (c) NO2 (d) SiO2
- Compounds are always (a) heterogeneous (b) homogeneous (c) homogeneous or heterogeneous (d) chemogeneous
THEORY
- (a) Define (i) Compound (ii) Mixture
(b)Give two examples each of compound and Mixtures
2. (a) State four differences between compound and mixture
(b) What is the formula of
- tetraoxosulphate
- Ammonium sulphide
- Sodium tetraoxophosphate acid
In our next class, we will be talking about Standard Separation Techniques for Mixtures. We hope you enjoyed the class.
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.

is sand a mixture or compound
A compound
Actually it is a Mixture. A heterogeneous mixture.
Mixture
Summary of compound and mixture is
sand is a compound it consists of silicon and oxygen
The note on the page is well explanatory and very good.
I enjoyed making use of this site to get the notes I needed .
Please, is this the current scheme of work for chemistry first term? Am sorry to ask, am seeing different scheme of work in someone’s note
how to write a molecular formula
Convert the mass of each element to moles using the molar mass from the periodic table. Then divide the given molecular molar mass by the molar mass calculated for the empirical formula Multiply each subscript by the whole number that resulted from step 2. This is now the molecular formula.
Nice
Nice. I love it
Thanks for the lessons
it is great
I really really enjoy this lesson and updated my notes
is your Igbo name chinememnma
Thank you Sir I now understand more on compound and mixtures
this is nice but I really need the separation techniques
Great
I need evaporation