Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m happy to see you ready to learn again. Let’s start with something simple. Imagine two neighbours who both want light at night, but instead of buying two separate lanterns, they decide to share one bright lantern between their houses. In Chemistry, some atoms prefer to share electrons instead of transferring them completely. This type of “sharing” is called covalent bonding. Today, we’ll look at how covalent bonds work, using the octet rule and Lewis structures to understand them.
Covalent Bonding: Lewis Structures, Octet Rule
What is Covalent Bonding?
Covalent bonding is the type of chemical bonding that occurs when two atoms share pairs of electrons to achieve stability.
Usually occurs between non-metal atoms.
The shared electrons help both atoms achieve a stable outer shell, often with 8 electrons (the octet rule).
The Octet Rule
The octet rule states that atoms tend to form bonds in such a way that each atom has 8 electrons in its valence (outermost) shell, similar to the stable noble gases.
Hydrogen is an exception; it is stable with just 2 electrons (duplet rule).
Lewis Structures
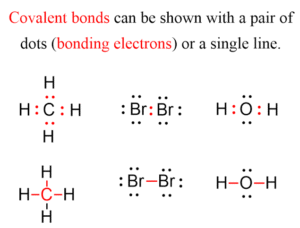
Lewis structures (also called electron dot structures) are simple diagrams that show how atoms share electrons in covalent bonding.
Dots represent valence electrons.
Shared pairs of electrons (bonding pairs) are placed between atoms.
Lone pairs (non-bonding pairs) remain on the individual atom.
Examples of Covalent Bonds
Hydrogen molecule (H₂):
Each hydrogen atom has 1 electron.
They share a pair of electrons, giving each atom 2 electrons (stable duplet).
Lewis structure: H : H
Oxygen molecule (O₂):
Each oxygen atom has 6 valence electrons.
They need 2 more electrons each to complete the octet.
They share 2 pairs of electrons (a double bond).
Lewis structure: O :: O
Water molecule (H₂O):
Oxygen has 6 valence electrons.
Each hydrogen contributes 1 electron.
Oxygen shares electrons with 2 hydrogens, forming 2 covalent bonds, achieving stability.
Carbon dioxide (CO₂):
Carbon has 4 valence electrons, oxygen has 6 each.
Carbon shares 2 electrons with each oxygen, forming double bonds.
Lewis structure: O :: C :: O
Properties of Covalent Compounds
Usually gases, liquids, or soft solids.
Low melting and boiling points (weak forces between molecules).
Do not conduct electricity (no free ions).
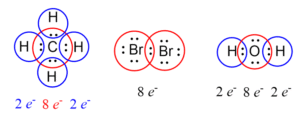
Often insoluble in water but soluble in organic solvents.
Everyday Connection
The oxygen you breathe (O₂), the water you drink (H₂O), and the carbon dioxide you exhale (CO₂) are all covalent compounds.
Plastics, petrol, and many medicines are also covalent in nature.
Summary
- Covalent bonding = sharing of electrons between non-metals.
- Octet rule: atoms aim to achieve 8 valence electrons for stability.
- Lewis structures show shared pairs (bonds) and lone pairs of electrons.
- Covalent compounds are usually gases or liquids with low melting points and poor conductivity.
Evaluation
- Define covalent bonding.
- State the octet rule.
- Draw the Lewis structures for H₂, O₂, and CO₂.
- Give two properties of covalent compounds.
Excellent work today! You’ve seen how atoms “share lanterns” (electrons) to achieve stability, and how Lewis structures make this sharing easy to picture. With Afrilearn, Chemistry becomes clear and fun—your success is certain. Keep your energy high for the next lesson!
