Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s great to have you back. Let me begin with something simple. Have you ever noticed that in some Nigerian families, a child may not resemble their immediate brother or sister, but surprisingly looks very much like a cousin? Chemistry has something similar—elements that don’t look like their immediate “neighbours” in the periodic table but share striking similarities with elements diagonally placed next to them. This special connection is called a diagonal relationship.
Diagonal Relationships
What is a Diagonal Relationship?
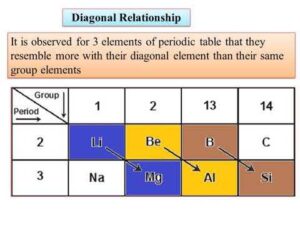
A diagonal relationship refers to the similarity in chemical properties between certain pairs of elements that are diagonally adjacent in the periodic table, especially in the second and third periods.
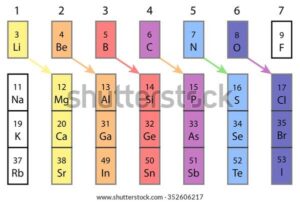
For example:
Lithium (Li) and Magnesium (Mg).
Beryllium (Be) and Aluminium (Al).
Why Do Diagonal Relationships Occur?
They occur because of a balance between two opposing trends:
Across a period: Atoms become smaller, electronegativity increases, and metallic character decreases.
Down a group: Atoms become larger, electronegativity decreases, and metallic character increases.
When you move diagonally (down one group and across one period), these effects tend to cancel each other out, making the elements show similar properties.
Examples of Diagonal Relationships
Lithium (Li) and Magnesium (Mg):
Both form nitrides (Li₃N and Mg₃N).
Both form sparingly soluble hydroxides and carbonates.
Both form soluble chlorides that crystallise with water molecules (LiCl·2H₂O, MgCl₂·6H₂O).
Beryllium (Be) and Aluminium (Al):
Both form amphoteric oxides and hydroxides (BeO and Al₂O₃ react with both acids and bases).
Both have a strong tendency to form covalent compounds.
Both form chlorides (BeCl₂ and AlCl₃) that act as Lewis acids.
Importance of Diagonal Relationships
They help explain exceptions to simple periodic trends.
They show how the periodic table is not only about vertical groups or horizontal periods but also about unique diagonal similarities.
They aid in predicting the chemistry of less familiar elements.
Everyday Connection
Think of it like schools in Nigeria: a student in SS1 at one school might behave more like a student in SS2 at another school due to similarities in teaching style, discipline, or culture, even though they are not in the same class or year group.
Summary
- Diagonal relationship = similarity in properties between elements diagonally placed in the periodic table.
- Common examples: Li & Mg, Be & Al.
- It happens because trends across periods and down groups balance out.
- Important in predicting properties of elements.
Evaluation
- Define diagonal relationship.
- Give two similarities between lithium and magnesium.
- Why does a diagonal relationship occur?
Fantastic job! You’ve learnt that Chemistry has “family resemblances” not just across rows and columns, but also diagonally. With Afrilearn, you are seeing the periodic table as a living, interconnected map of relationships. Keep learning—you’re becoming a true master of patterns in Chemistry!
