Back to: CHEMISTRY SS2
Welcome to Class !!
We are eager to have you join us !!
In today’s Chemistry class, We will be learning about Oxidizing and Reducing Agents. We hope you enjoy the class!
CONTENT
- Identification of Oxidizing and Reducing agents
- Balancing of Redox equation in Acidic and Alkaline medium
OXIDIZING AND REDUCING AGENTS
An oxidizing agent is defined as a substance which loses oxygen or electronegative element to another substance. Or an oxidizing agent is a substance which gains hydrogen from a substance. Or an oxidizing agent is a substance which gains an electron from a substance. Consider the reaction below
C(s) + ZnO(s) CO2(g) + Zn(s)
ZnO is the oxidizing agent because it loses oxygen to C.
A reducing agent is defined as a substance, which removes and accepts oxygen from other substances. Or a reducing agent is defined as a substance, which removes and accepts the electronegative element from another substance. Or a reducing agent is defined as a substance which loses and donates an electron to another substance. From the reaction above, C is the reducing agent because it removes and accepts oxygen from ZnO.
In an oxidation and reduction reaction, the oxidizing agent is the reduced species while the reducing agent is the oxidized species.
NOTE: An oxidizing agent accepts an electron, is reduced and its oxidation number decreases while a reducing agent donate electron, is oxidized and its oxidation number increases.
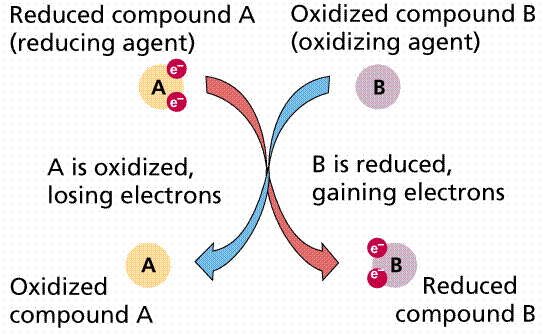
IDENTIFICATION OF OXIDIZING AND REDUCING AGENTS
TESTS FOR OXIDIZING AGENTS: The presence of an oxidizing agent can be detected using any of the following reagents.
- Acidified potassium iodide, KI with starch
- Sulphur (IV) oxides, SO2 with acidified Barium trioxonitrate (V) solution
- Iron (II) Chloride solution (FeCl2)
- Hydrogen sulphide gas (H2S)
SUMMARY OF TEST
| S/NO | TEST | OBSERVATION | INFERENCE |
| 1 | O.A + FeCl2(aq) | Green colour of Fe2+ solution turns to reddish-brown of Fe3+ | O.A is present
|
| 2 | O.A + H2S(g) | Formation of yellow deposits of sulphur | O.A is present |
| 3a.
b. | O.A + acidified KI
Red- brown solution + starch | Reddish-brown colouration produced. Iodine is liberated. Reddish-brown turns dark blue. The iodine reacts with the starch | O.A is present |
| 4 | O.A + SO2(g) + dilute HNO3(aq) + Ba(NO3)2(aq) | A white precipitate of insoluble BaSO4 is formed | O.A is present
|
TEST FOR REDUCING AGENTS: Reducing agent is detected in the laboratory using any of the following reagents.
- Acidified Potassium tetraoxomanganate (VII)
- Acidified Potassium heptaoxodichromate (VI)
| S/N | TEST | OBSERVATION | INFERENCE |
| 1 | R.A + acidified KMO4 | The purple solution of KMnO4 turns colourless on the addition of R.A | R.A is present |
| 2 | R.A + acidified K2Cr2O7 | The orange solution of K2Cr2O7 turns green solution addition of R.A | R.A is present |
Common oxidizing agents are concentrated HNO3, H2SO4, KMnO4, K2Cr2O7, O2, Cl2 etc.
Common reducing agents are: concentrated HCl, pure metals, carbon, H2, SO2, H2S, etc.
EVALUATION
- Define Oxidizing agent and Reducing agent in terms of electron transfer
- Describe one test each for identifying an Oxidizing agent and a Reducing agent
BALANCING OF REDOX EQUATIONS
Redox equations are balanced by first considering the two half equations involved in such reaction. Steps involved are
- Identify the oxidizing and reducing agents and deduce expected products.
- Write the half equations for oxidation and reduction. Balance the atoms and charges for each equation.
- Make sure that the loss of the electron in the oxidation half equation is balanced by the electrons gain in the reduction half equation.
- Combine the halves equations to eliminate the electrons and get the overall redox equation.
EXAMPLE 1: Write a balanced ionic equation for the redox reaction between potassium tetraoxomanganate(VII) and Iron (II)tetraoxosulphate(VI)in acidic medium.
SOLUTION:
O.A MnO4–
R.A Fe2+
OXIDATION HALF EQUATION
Fe2+ Fe3+ + e–
REDUCTION HALF EQUATION
MnO4– + H+ Mn2+ + H2O
BALANCED HALF EQUATIONS
5Fe2+ Fe3+ + 5e–
MnO4– + 8H++ 5e– Mn2+ + 4H2O
COMBINED EQUATION
5Fe2+ + MnO4– +8H+ + 5e– Fe3++ 5e– + Mn2+ +4H2O
The electrons on both sides of the equation cancel out and the overall equation is
5Fe2+ + MnO4– + 8H+ Fe3+ + Mn2+ + 4H2O
EXAMPLE 2: Write a balanced equation for the following reaction in basic medium
Cr3+ + BrO– CrO42- + Br–
SOLUTION:
O.A BrO–
R.A Cr3+
OXIDATION HALF EQUATION
Cr3+ CrO42-
Balancing of atoms: Cr3+ + 8OH– CrO42- + 4H2O
Balancing of charges: Cr3+ + 8OH– CrO42- + 4H2O + 3e–
REDUCTION HALF EQUATION
BrO– Br–
Balancing the atoms: BrO– + H2O Br– + 2OH–
Balancing the charges: BrO– + H2O + 2e– Br– + 2OH–
But electron lost in the oxidation half must equal electron gained in the reduction half equation.
Multiplying the oxidation half equation by 2 and the reduction half equation by 3 gives
BALANCED HALF EQUATIONS
2Cr3+ +16OH– 2CrO42- + 8H2O + 6e–
3BrO– + 3H2O + 6e– 3Br– + 6OH–
COMBINED EQUATION
2Cr3+ + 16OH– + 3BrO– + 3H2O + 6e– 2CrO42- + 8H2O + 6e– + 3Br– + 6OH–
The electrons on both sides of the equation cancel out and the overall equation is
2Cr3+ + 16OH– + 3BrO– + 3H2O 2CrO42- + 8H2O + 3Br– + 6OH–
GENERAL EVALUATION/REVISION
- Determine the oxidation number of
(a) Fe in Fe2O3 (b) Cu in [Cu(NH3)4]2+
- Name the following compounds
(a) H2CO3 (b) KMnO4
- The compound Na2S is called ————–
- The IUPAC name of NaHSO4 is————-
- Balance the following redox equation:
I– + MnO4– IO3– + MnO2 in basic medium
READING ASSIGNMENT
New School Chemistry for Senior Secondary School by O. Y. Ababio, pages 193-196
WEEKEND ASSIGNMENT
SECTION A: Write the correct option ONLY
1 What is the value of x in the following equation?
Cr2O72-+ 14H+ + xe- 2Cr3+ + 7H2 O
A. 1 B. 6 C. 8 D. 12
2 In which of the following is the oxidation number of sulphur equal to -2?
A. S8 B. H2S C. SO2D. SO3.2-
3 Which species undergo a reduction in the reaction represented by the equation below?
H2S(g) + 2FeCl3(aq) S(s) +2HCl(aq) + 2FeCl2(aq)
A. Fe3+ B. H2S C. Cl– D. S
4 Cr2O72- + 6Fe2++ 14H+ = 2Cr 3+ + 6Fe3+ + 7H2O In the equation above, the oxidation number of chromium changes from
A. +7 to +3 B. +6 to +3 C. -6 to +3 d. -2 to +6
5 When SO2 is passed into a solution of acidified potassium heptaoxodichromate(VI) (K2Cr2O7), the solution turns A. green B. orange C. purple d. yellow
SECTION B
- Determine the oxidation state of P in each of the following structure
- POCl3 b. PH3
- Balance the following redox equation: Cr2O72- + SO2 Cr3+ + SO42- in acidic medium
We have come to the end of this class. We do hope you enjoyed the class?
Should you have any further question, feel free to ask in the comment section below and trust us to respond as soon as possible.
In our next class, we will be learning about Ionic Theory. We are very much eager to meet you there.

Examples of oxidizing and reducing agent