Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m excited to see you today. Let me ask: have you ever watched football in a big stadium like the Abuja National Stadium? Each spectator has a particular seat, in a particular row, in a particular section. No two spectators can sit in exactly the same spot at the same time. In the same way, electrons in an atom each have a unique “address.” This address is described by something we call quantum numbers.
Quantum Numbers
What are Quantum Numbers?
Quantum numbers are numbers that describe the position, energy, and spin of an electron in an atom. They help us know exactly where each electron is located—just like a house address helps you find where someone lives. There are four quantum numbers: principal (n), azimuthal (l), magnetic (m), and spin (s).
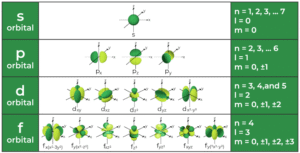
Principal Quantum Number (n)
This tells us the main energy level or shell where the electron is found. Think of it as the street on which the house is located. It can take values like 1, 2, 3, and so on. The higher the number, the farther the electron is from the nucleus and the more energy it has. For example, electrons in n = 1 are closest to the nucleus, while those in n = 4 are farther away.
Azimuthal Quantum Number (l)
This describes the shape of the orbital the electron occupies. Think of it like the type of house on the street: is it a bungalow, duplex, or flat? The values depend on n. If n = 3, then l can be 0, 1, or 2. Each value corresponds to orbital shapes:
l = 0 → s orbital (spherical)
l = 1 → p orbital (dumbbell-shaped)
l = 2 → d orbital (clover-shaped)
l = 3 → f orbital (complex shape)
Magnetic Quantum Number (m)
This tells us the orientation of the orbital in space. Think of it like the exact apartment number in the building. Its values range from –l to +l. For instance, if l = 1 (p orbital), then m can be –1, 0, or +1, which means there are three p orbitals oriented differently in space.
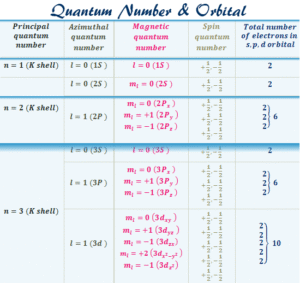
Spin Quantum Number (s)
Electrons are not just sitting still—they spin, like a football rolling on the ground. The spin quantum number tells us the direction of this spin, which can be +½ or –½. No two electrons in the same orbital can have the same spin; one spins up, and the other spins down.
Summary
- Principal quantum number (n): main energy level.
- Azimuthal quantum number (l): shape of the orbital.
- Magnetic quantum number (m): orientation of orbital in space.
- Spin quantum number (s): direction of electron spin.
Evaluation
- Which quantum number tells you the shape of an orbital?
- If n = 2, what are the possible values of l?
- What are the two possible values of the spin quantum number?
You are doing excellently well! Remember, just like every spectator in a stadium has a unique seat, every electron in an atom has its unique set of quantum numbers. Keep building your confidence—Chemistry is your ticket to seeing the hidden order in the world around you. With Afrilearn, you’re always one step ahead.
