Back to: Botany 200 Level
Hello, my brilliant student! I hope you’re having a great day! Have you ever wondered why some plants thrive in certain soils while others struggle? One key reason is soil pH. Just like our bodies need the right balance of nutrients to stay healthy, plants also need a balanced soil pH to absorb nutrients properly. Today, we’ll explore how soil pH affects nutrient availability and what happens when the balance is off.
Role of Soil pH in Nutrient Availability
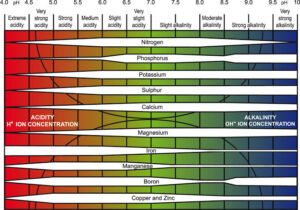
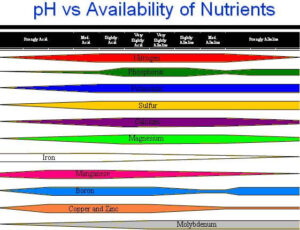
Soil pH is a measure of how acidic or alkaline the soil is. It is measured on a scale of 0 to 14:
Acidic soil → pH below 6.5 (e.g., rainforest soils, peaty soils).
Neutral soil → pH around 7 (ideal for most plants).
Alkaline soil → pH above 7.5 (e.g., desert soils, limestone-rich soils).
Soil pH is important because it controls how easily plants can absorb nutrients. If the pH is too high or too low, some nutrients become unavailable, even if they are present in the soil.
How Soil pH Affects Nutrient Availability
At Low pH (Acidic Soil, pH < 6.5)
Nutrients like iron (Fe), manganese (Mn), and aluminium (Al) become too available, leading to toxicity.
Essential nutrients like phosphorus (P), calcium (Ca), and magnesium (Mg) become less available, causing deficiencies.
Microbial activity slows down, reducing nitrogen availability.
At High pH (Alkaline Soil, pH > 7.5)
Nutrients like iron (Fe), zinc (Zn), and phosphorus (P) become locked up, making them unavailable to plants.
Micronutrient deficiencies (especially iron and zinc) occur, leading to yellowing leaves.
Microbial activity decreases, reducing the breakdown of organic matter.
At Neutral pH (6.0 – 7.5, Best Range for Most Plants)
Most nutrients are available in the right amounts.
Microbial activity is high, helping plants access nitrogen and organic nutrients.
Plants grow healthier and stronger because they can absorb all the nutrients they need.
How to Adjust Soil pH for Better Nutrient Availability
To Reduce Soil Acidity (Increase pH)
Add lime (calcium carbonate) to neutralise excess acidity.
Use wood ash or dolomite to provide calcium and magnesium.
Plant legumes to improve nitrogen availability.
To Reduce Soil Alkalinity (Lower pH)
Add sulphur or aluminium sulphate to make the soil more acidic.
Use organic matter like compost to increase microbial activity.
Apply acidic fertilisers (e.g., ammonium sulphate) to balance pH.
Why Is This Important?
Farmers and gardeners must check soil pH before planting to ensure the soil has the right conditions for crops.

If plants lack nutrients despite fertiliser application, soil pH might be the problem.
Correcting soil pH improves plant health, increases yields, and prevents nutrient waste.
Summary
Soil pH controls how easily plants can absorb nutrients.
Acidic soils (low pH) make some nutrients too available (toxic) and others unavailable.
Alkaline soils (high pH) cause micronutrient deficiencies, especially iron and phosphorus.
Neutral soil (pH 6.0 – 7.5) is the best range for most plants.
Farmers can adjust soil pH using lime (to increase pH) or sulphur (to lower pH).
Evaluation
- What is the best soil pH range for most plants?
- What happens to iron and phosphorus in acidic soil?
- How can farmers reduce soil acidity to improve nutrient availability?
- Why do plants in alkaline soil often suffer from yellow leaves?
- What role do microorganisms play in maintaining soil pH?
Great job! Now you understand how soil pH affects plant nutrition and how to manage it for healthy plant growth. Keep learning—Afrilearn is here to make learning simple and fun! See you in the next lesson!
