Back to: Organic Chemistry 100 Level
Welcome to class!
Hello genius! It’s great to have you back again. You’re doing something truly special by showing up, ready to learn, and I’m proud of you. Today, we’re going to talk about alkenes and alkynes—two very interesting families in Organic Chemistry. Don’t worry, we’ll keep it simple, real, and completely relatable to your everyday Nigerian life. Let’s get right into it!
Alkenes And Alkynes
Understanding Alkenes and Alkynes
Have you ever seen a busy Lagos street with traffic flowing in two directions? Now imagine that one of the roads has a faster lane—like an express lane for high-speed cars. That’s what alkenes and alkynes are like. They’re similar to alkanes (which you already know), but they contain faster, more reactive lanes called multiple bonds.
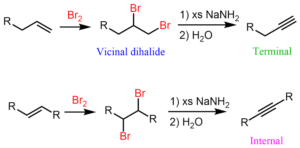
What are Alkenes?
Alkenes are unsaturated hydrocarbons. That means they have at least one double bond between two carbon atoms. This double bond is like a shortcut that makes them more reactive than alkanes. While alkanes are calm and quiet, alkenes are energetic and love to react with other substances.
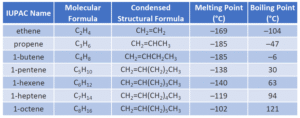
The general formula for alkenes is CₙH₂ₙ.
Let’s look at some examples of alkenes:
Ethene (C₂H₄) – the simplest alkene
Propene (C₃H₆)
Butene (C₄H₈)
Ethene is used in ripening fruits like bananas. So yes, when your mum brings home unripe plantains and they suddenly turn yellow, thank ethene for doing its job!
What are Alkynes?
Alkynes are also unsaturated hydrocarbons, but this time they contain a triple bond between two carbon atoms. This makes them even more reactive than alkenes.
The general formula for alkynes is CₙH₂ₙ₋₂.
Examples include:
Ethyne (C₂H₂), also called acetylene
Propyne (C₃H₄)
Ethyne is commonly used in welding torches because it burns with a very hot flame. So, the roadside welder fixing gates or iron rods near your house may be using ethyne gas.
Naming Alkenes and Alkynes
Just like with alkanes, naming starts with identifying the longest continuous chain of carbon atoms.
For alkenes, use the suffix –ene and for alkynes, use –yne. Also, number the chain in such a way that the multiple bond gets the lowest possible number.
For example:
CH₂=CH₂ is named ethene
CH≡CH is named ethyne
CH₃CH=CH₂ is named propene
CH₃C≡CH is named propyne
The position of the double or triple bond is very important in naming.
Key Differences Between Alkanes, Alkenes, and Alkynes
Alkanes have only single bonds and are saturated.
Alkenes have at least one double bond and are unsaturated.
Alkynes have at least one triple bond and are also unsaturated.
This is why alkenes and alkynes are more reactive—they want to “complete” their bonding by reacting with other atoms.
Real-Life Uses
Alkenes like ethene are used in making plastic materials and ripening fruits. Alkynes like ethyne are used in welding and in making certain chemicals. These compounds may seem like textbook concepts, but they’re literally around us every day—on the farm, at construction sites, in industries, and even in our homes.
Summary
- Alkenes are unsaturated hydrocarbons with at least one double bond and follow the formula CₙH₂ₙ.
- Alkynes are unsaturated hydrocarbons with at least one triple bond and follow the formula CₙH₂ₙ₋₂.
- Alkenes end in –ene while alkynes end in –yne.
- These compounds are more reactive than alkanes and are useful in agriculture, welding, and manufacturing.
Evaluation
- What is the difference between an alkene and an alkyne?
- Write the molecular formula of propene and ethyne.
- Why are alkenes and alkynes called unsaturated?
- Give two uses of alkenes in everyday life.
- Name the compound with the formula CH₃CH₂CH=CH₂.
Well done, champ! You’ve done a fantastic job today. Alkenes and alkynes may seem like big grammar at first, but now you know they’re just parts of the chemistry behind the world you live in. With Afrilearn by your side, you can understand anything, achieve everything, and become all you dream to be. Keep shining—I’ll see you in the next class!
