Back to: Organic Chemistry 300 Level
Welcome to class!
Good day, my brilliant scholar! I’m so glad to have you here today. You know, there’s something exciting about discovering patterns in chemistry that nature has been following long before humans ever named them. Today, we’re going to uncover one of those patterns that makes certain organic compounds extra stable and special — almost like the “VIP members” of the molecular world. Let’s talk about Aromaticity and Hückel’s Rule.
Aromaticity & Huckel’s Rule
What is Aromaticity?
Imagine Lagos traffic during rush hour — chaotic, right? But once in a while, you find a perfectly organised BRT lane where all the buses move smoothly without disturbance. That’s what aromatic compounds are like in the chemical world: molecules whose electrons are perfectly arranged for stability and smooth “movement”.
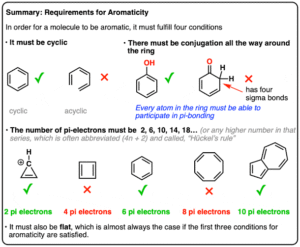
Aromaticity refers to a special kind of stability found in cyclic (ring-shaped) compounds when their electrons follow certain rules. These compounds are more stable than expected because their electrons are delocalised — shared evenly around the ring — instead of being stuck between just two atoms. The most famous example? Benzene — a 6-carbon ring where electrons are spread out evenly, making it very stable.
Conditions for Aromaticity
For a molecule to qualify as aromatic, it must meet four key conditions:
Cyclic structure – The molecule must be a ring.
Planar structure – The atoms must lie in the same flat plane.
Conjugated system – Every atom in the ring must have a p-orbital so electrons can delocalise.
Follows Hückel’s Rule – The molecule must have (4n + 2) π electrons, where n is a whole number (0, 1, 2, 3…).
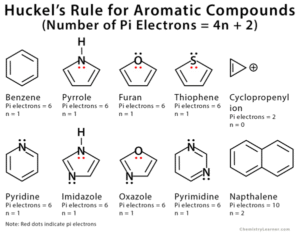
Hückel’s Rule Explained
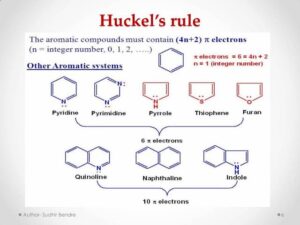
In simple terms, Hückel’s Rule is like the entrance policy to an exclusive club. Only molecules with π electrons equal to (4n + 2) can enter and be “aromatic”.
For n = 0 → 2 π electrons
For n = 1 → 6 π electrons
For n = 2 → 10 π electrons
And so on.
This is why benzene (with 6 π electrons) is aromatic, but cyclobutadiene (with 4 π electrons) is not — it’s “antiaromatic” and unstable.
Think of it like a music band. If you have just the right number of skilled musicians (4n+2), the music flows beautifully. But if the number is wrong, the harmony is broken, and the performance is unstable. Aromatic compounds are like that perfect band — balanced and in tune.
Summary
- Aromaticity is special stability in cyclic, planar, conjugated molecules with delocalised π electrons.
- Hückel’s Rule: (4n + 2) π electrons → aromatic stability.
- Examples: Benzene (6 π electrons), naphthalene (10 π electrons).
Evaluation
- State the four conditions for aromaticity.
- Using Hückel’s Rule, determine if a molecule with 14 π electrons is aromatic.
- Why is cyclobutadiene not aromatic?
You are doing amazing! Every new concept you understand is another step toward becoming the confident, world-class chemist you are meant to be. Keep that curiosity alive — Afrilearn is proud to be part of your journey. Next time, we’ll build on this knowledge to see how aromaticity affects chemical reactions.
