Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant learner! I hope you’re ready for another exciting journey into the world of aromatic compounds. You know how in our daily lives, some places are so popular that people keep coming to visit again and again — like Balogun Market in Lagos or Ariaria Market in Aba? Well, aromatic rings are like that too. They have a way of attracting certain “visitors” called electrophiles, and these guests always seem to come for the same type of “visit” — substitution. Today, we’ll discover exactly how this happens.
Electrophilic Substitution In Aromatics I
What is Electrophilic Substitution in Aromatics?
Aromatic compounds, like benzene, are highly stable because of their delocalised π electrons. If we try to add something to them directly (like in alkenes), we would destroy that stability — and they don’t like that at all. Instead, they prefer to substitute one of their hydrogen atoms for a new group, keeping their aromatic stability intact.

The “electrophilic” part simply means the visitor is electron-loving. These are positively charged or electron-deficient species that are attracted to the rich π electron cloud of aromatic rings.
General Mechanism
The process happens in three main steps:
Generation of the electrophile
This is like preparing the guest before they enter the aromatic ring’s “market”.
For example, in nitration, concentrated nitric acid and sulphuric acid react to produce the nitronium ion (NO₂⁺) — the electrophile.
Attack of the electrophile (formation of the arenium ion)
The π electrons from the aromatic ring temporarily attack the electrophile, forming a positively charged intermediate called the arenium ion (or sigma complex).
This is the only time aromaticity is lost, so the ring is in a hurry to get back to its stable state.
Restoration of aromaticity
A base removes a proton (H⁺) from the intermediate, and the π electron system is restored — the aromatic ring is happy again, with a new group in place of the hydrogen.
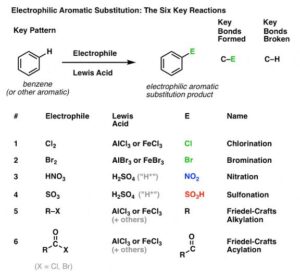
Common Examples
Nitration – introducing a nitro group (-NO₂) using concentrated HNO₃/H₂SO₄.
Sulfonation – adding a sulfonic acid group (-SO₃H) using concentrated H₂SO₄ or fuming H₂SO₄.
Halogenation – adding a halogen (Cl or Br) using halogen plus a Lewis acid catalyst like FeCl₃ or FeBr₃.

Think of the aromatic ring as a popular celebrity compound. Instead of letting new people completely change their style (like in addition reactions), they just swap accessories — replacing a hat for sunglasses — while keeping their core look.
Summary
- Electrophilic substitution keeps the aromatic stability intact.
- Steps: Generate electrophile → Attack on aromatic ring → Restore aromaticity.
- Examples include nitration, sulfonation, and halogenation.
Evaluation
- Why do aromatic compounds prefer substitution over addition reactions?
- List and explain the three main steps in the electrophilic substitution mechanism.
- Give one example of electrophile generation for halogenation.
You’ve just mastered the basics of electrophilic substitution — one of the most important reaction types in aromatic chemistry. Keep going; your understanding is growing stronger every day. Afrilearn is proud to see you becoming a confident chemistry thinker ready for global success.
