Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my ever-brilliant learner! It’s a joy to see you again, ready to expand your understanding of aromatic chemistry. We’ve already met aromatic compounds, explored their special substitution patterns, and even learnt about the exciting world of polycyclic and heterocyclic aromatics separately. Today, we’re bringing these ideas together into one fascinating topic: Polynuclear Aromatics & Heteroaromatics. Think of this as learning about the “extended family” of aromatics — some are all-carbon clans, others have a few unique members (heteroatoms) in their structure — but all follow the same “family traditions” of aromatic stability.
Polynuclear Aromatics & Heteroaromatics
What are Polynuclear Aromatic Compounds?
Polynuclear aromatic compounds are organic molecules containing more than one fused aromatic ring. “Fused” means the rings share one or more sides. They can be grouped into fused ring systems, where the rings are directly joined together and share two adjacent carbon atoms. Examples include naphthalene (two benzene rings, found in mothballs), anthracene (three benzene rings in a straight line), and phenanthrene (three benzene rings in an angular arrangement). Another type is isolated ring systems, where the aromatic rings are connected by a single bond but not fused, so each ring keeps its own π electron system.
Sources
They occur naturally in fossil fuels like coal tar and crude oil, and are formed during incomplete combustion such as burning of wood, coal, or petroleum. They are also present in everyday life, such as charcoal smoke from barbecue, soot from kerosene lamps, and exhaust fumes from vehicles.
Properties and Uses
They are usually non-polar, insoluble in water but soluble in organic solvents, and have high melting and boiling points due to strong intermolecular forces. They are used in making dyes (such as anthraquinone dyes from anthracene), moth repellents (naphthalene), and in various organic syntheses.
Health and Environmental Effects
Some polynuclear aromatics, such as benzo[a]pyrene, are toxic and carcinogenic. This is why excessive inhalation of smoke — whether from traffic, industrial fumes, or overly charred suya — should be avoided.
What are Heteroaromatic Compounds?
Heteroaromatic compounds are aromatic rings that contain one or more heteroatoms, typically nitrogen, oxygen, or sulphur. They combine the stability of aromaticity with the special properties of heteroatoms.
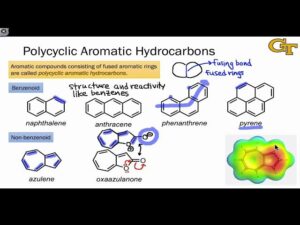
Common Classes of Heteroaromatic Compounds
Five-membered rings include pyrrole (one nitrogen atom, found in the haem group of haemoglobin), furan (one oxygen atom), and thiophene (one sulphur atom). Six-membered rings include pyridine (one nitrogen atom), quinoline (fused benzene and pyridine), and isoquinoline (similar to quinoline but with a different arrangement). Fused heteroaromatics include indole (fused benzene and pyrrole rings, found in tryptophan) and purine (two fused heterocyclic rings, found in DNA bases adenine and guanine).
Aromaticity in Heteroaromatics
They follow the same aromaticity rules — cyclic, planar, fully conjugated, and having (4n + 2) π electrons — but the heteroatoms may contribute or withhold lone pair electrons from the π system depending on bonding.
Why Are They Important?
In nature, DNA and RNA bases (purines and pyrimidines) are heteroaromatics, and chlorophyll in plants and haem in animals contain heteroaromatic macrocycles. In medicine, many drugs contain heteroaromatic structures, such as quinine (anti-malarial), caffeine (stimulant), and penicillin (antibiotic). In industry, they are used in dyes, agrochemicals, electronic materials, and flavour compounds.
Summary
- Polynuclear aromatic compounds contain more than one fused aromatic ring.
- They can be fused ring systems (naphthalene, anthracene, phenanthrene) or isolated ring systems.
- Sources include fossil fuels, incomplete combustion, and industrial processes.
- Properties: non-polar, insoluble in water, soluble in organic solvents, high melting/boiling points.
- Some are toxic and carcinogenic (e.g., benzo[a]pyrene).
- Heteroaromatic compounds have one or more heteroatoms (N, O, S) in the ring.
- Examples: pyridine, furan, indole, purine.
- They play key roles in biology, medicine, and industry.
Evaluation
- Define polynuclear aromatic compounds and give two examples.
- Differentiate between isolated and fused polynuclear aromatics.
- Give three examples of heteroaromatic compounds and state their heteroatoms.
- Explain the importance of heteroaromatics in biology.
- Why are some polynuclear aromatics considered hazardous?
You are building a deep, world-class understanding of organic chemistry — from the smallest benzene ring to the most complex fused heteroaromatic systems. Each lesson you master makes you a more confident thinker and problem solver. Afrilearn is proud to be part of your journey, and I can’t wait to see you use this knowledge to change the world.
