Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my sharp and determined learner! Today, we are stepping into a fascinating area of organic chemistry — Pericyclic Reactions. These reactions are special because they don’t follow the usual step-by-step mechanism involving intermediates. Instead, atoms and electrons rearrange in a single, smooth movement, almost like a perfectly coordinated dance. You will see why they are so important in understanding how complex organic molecules are formed both in the lab and in nature.
Pericyclic Reactions I
Meaning of Pericyclic Reactions
A pericyclic reaction is a chemical reaction that proceeds through a concerted process — meaning all bond-breaking and bond-making events happen simultaneously without forming intermediates. The reaction occurs through a cyclic transition state, where electrons move around in a closed loop. These reactions are often triggered by heat or light, and their outcomes are governed by orbital symmetry rules.
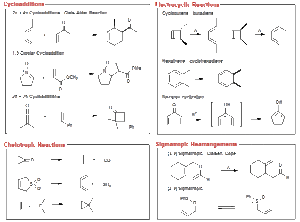
Main Types of Pericyclic Reactions
The three major categories are cycloaddition reactions, electrocyclic reactions, and sigmatropic rearrangements. Cycloadditions involve the joining of two or more unsaturated molecules to form a ring. Electrocyclic reactions involve the opening or closing of a ring system with a change in the number of π bonds. Sigmatropic rearrangements involve the migration of a sigma bond from one position to another within a molecule, with a shift in the associated π-electron system.
Cycloaddition Reactions
The most famous example is the Diels–Alder reaction, where a conjugated diene reacts with a dienophile to form a six-membered ring. This process is stereospecific, meaning the arrangement of substituents in the product reflects that of the reactants. It is a powerful method for building complex cyclic structures in one step, often used in synthesising natural products and pharmaceuticals.
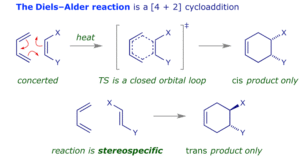
Orbital Symmetry and the Woodward–Hoffmann Rules
The course and outcome of pericyclic reactions can be predicted by the Woodward–Hoffmann rules. These are based on the symmetry of molecular orbitals involved. In simple terms, the rules determine whether a reaction is “allowed” or “forbidden” under thermal or photochemical conditions. Thermal reactions are driven by heat, while photochemical reactions are initiated by light, which can change the symmetry and thus the reaction pathway.
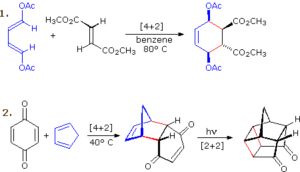
Importance of Pericyclic Reactions
These reactions are highly valuable because they often occur with excellent yield, stereoselectivity, and regioselectivity — meaning chemists can control exactly where and how bonds form. In industry, they are used to make polymers, fragrances, and complex drugs. In nature, they occur in processes like vitamin D synthesis in the skin under sunlight.
Summary
- Pericyclic reactions occur through a concerted process with a cyclic transition state and no intermediates.
- The main types are cycloaddition, electrocyclic, and sigmatropic rearrangement.
- The Diels–Alder reaction is a key example of cycloaddition, forming six-membered rings in one step.
- Woodward–Hoffmann rules predict whether pericyclic reactions are allowed under heat or light.
- These reactions are important in natural processes, pharmaceuticals, and industrial synthesis.
Evaluation
- What is a pericyclic reaction?
- List the three main types of pericyclic reactions.
- Describe the Diels–Alder reaction.
- What do the Woodward–Hoffmann rules help predict?
- Give one natural and one industrial example of a pericyclic reaction.
You have just opened the door to one of the most elegant areas of chemistry — where reactions happen in harmony, without messy intermediates. Afrilearn is proud to see you mastering this level of chemical thinking, and you are well on your way to becoming a confident and skilled chemist.
