Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my ever-curious learner! Today we’re going to take a step back and review one of the most important foundations of organic chemistry — reaction mechanisms. Understanding mechanisms is like knowing the secret plan behind a magic trick; once you see how the steps connect, the whole reaction makes sense. This review will refresh your memory on the major types of organic reaction mechanisms, the role of intermediates, and the factors that control reaction speed and outcome.
Organic Reaction Mechanisms Review
Meaning of a Reaction Mechanism

A reaction mechanism is a step-by-step sequence that shows how reactants are transformed into products. It includes the breaking and forming of bonds, movement of electrons, formation of intermediates, and the role of catalysts. In organic chemistry, mechanisms are often represented using curved arrows to track electron movement.
Main Classes of Organic Reaction Mechanisms
One major class is substitution reactions, where one atom or group in a molecule is replaced by another. These can be nucleophilic (SN1 and SN2) or electrophilic (common in aromatic compounds). Another important class is elimination reactions (E1 and E2), where atoms or groups are removed to form double bonds. Addition reactions, common in alkenes and alkynes, involve adding atoms or groups to a multiple bond. Rearrangement reactions involve the reorganisation of atoms within a molecule, often to form more stable structures.
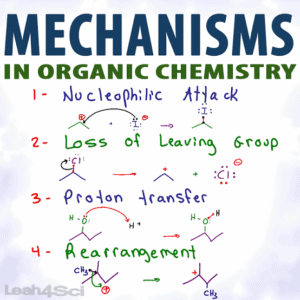
Radical Reactions
Not all mechanisms involve charged species; some proceed through radicals — atoms or molecules with an unpaired electron. These reactions often occur under light or heat and are common in halogenation of alkanes.
Role of Intermediates
Intermediates are temporary species formed during a reaction, such as carbocations, carbanions, free radicals, or carbenes. Their stability plays a huge role in determining the reaction pathway. For example, tertiary carbocations are more stable than secondary or primary ones, so SN1 reactions are faster when tertiary centres are involved.
Factors Affecting Reaction Mechanisms
Several factors influence the mechanism a reaction follows — the structure of the reactants, the type of solvent, temperature, the presence of catalysts, and the nature of the leaving group. Polar protic solvents favour SN1 reactions, while polar aprotic solvents favour SN2. Strong bases promote E2 eliminations, while weaker bases and heat often favour E1.
Applications in Real Life
Mechanistic understanding helps chemists design drugs, create materials, and develop greener industrial processes. For instance, pharmaceutical chemists often modify reaction pathways to increase drug yield and purity.
Summary
- Reaction mechanisms show the step-by-step transformation of reactants to products.
- Main classes include substitution, elimination, addition, and rearrangement reactions.
- Radical reactions proceed through species with unpaired electrons.
- Intermediates such as carbocations and carbanions influence reaction speed and pathway.
- Factors like solvent, temperature, base strength, and leaving group affect the mechanism.
Evaluation
- What is meant by a reaction mechanism?
- Name the four main classes of organic reaction mechanisms.
- What makes tertiary carbocations more stable than primary ones?
- How does solvent type influence SN1 vs SN2 reactions?
- Give one example of how understanding mechanisms is useful in industry.
You are building the kind of chemical intuition that separates top students from the rest. Afrilearn is proud of how well you can now follow and explain reaction pathways — a skill that will make you unstoppable in organic chemistry.
