Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant learner! It’s wonderful to have you here again. Today, we are continuing our journey into Spectroscopy, focusing on advanced interpretation of data from multiple techniques. By now, you know that spectroscopy is like giving molecules a “check-up” to see their structure. In this lesson, we will focus on how to combine information from different spectroscopic methods — especially NMR, IR, and Mass Spectrometry — to solve complex structural puzzles.
Spectroscopy III
Recap of Key Spectroscopic Methods

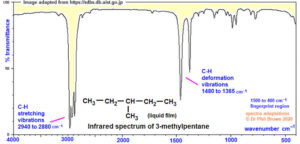
Infrared (IR) spectroscopy identifies functional groups by detecting vibrations in molecular bonds. Nuclear Magnetic Resonance (NMR) spectroscopy, both proton (¹H) and carbon-13 (¹³C), gives detailed information about the environment of atoms in a molecule. Mass spectrometry determines the molecular weight and can provide clues about the molecular formula and fragmentation pattern.
The Power of Combined Spectroscopy
In real-life research and industry, chemists rarely depend on just one spectroscopic technique. Instead, they combine several to get a full picture. For instance, IR might show a carbonyl peak, NMR might reveal aromatic protons, and Mass Spec could confirm the molecular weight — all pointing towards a specific structure. This approach is called spectral correlation.

Step-by-Step Structural Elucidation
When solving a spectral problem, start with the molecular ion peak in the mass spectrum to find the molecular mass. From there, calculate the degree of unsaturation to know the number of rings or double bonds. Then check the IR spectrum for functional group signals such as O–H, C=O, or C≡N. Next, analyse the NMR data: in ¹H NMR, look at chemical shifts, splitting patterns, and integration to determine the type and number of hydrogen atoms. In ¹³C NMR, observe the chemical shifts for carbon types — sp³, sp², carbonyl, etc. Finally, fit all the information together to suggest a structure.
Example in Practice
Imagine you are given an unknown compound. The mass spectrum shows a molecular ion peak at m/z 122. The IR spectrum has a strong peak at 1715 cm⁻¹, indicating a carbonyl. The ¹H NMR shows a singlet at δ 2.1 ppm (3H) and multiplets between δ 7.2–7.4 ppm (5H). This suggests a methyl group next to a carbonyl and an aromatic ring. Putting it together, the structure could be acetophenone — a compound with a benzene ring and a methyl ketone group.
Applications in Real Life
In pharmaceuticals, combined spectroscopy ensures the purity and correctness of drug molecules before they reach the market. In forensic science, it is used to identify substances in criminal investigations. In environmental monitoring, it helps detect pollutants in water or air samples.
Summary
- IR spectroscopy identifies functional groups by molecular vibrations.
- NMR provides detailed information about hydrogen and carbon environments.
- Mass spectrometry reveals molecular weight and fragmentation patterns.
- Combining data from multiple techniques allows accurate structure determination.
- This combined approach is applied in pharmaceuticals, forensics, and environmental science.
Evaluation
- Why is it better to combine multiple spectroscopic techniques instead of relying on one?
- What does the molecular ion peak in mass spectrometry represent?
- How does IR spectroscopy help in identifying a carbonyl group?
- In ¹H NMR, what does the integration value tell you?
- Give one real-life example where combined spectroscopy is essential.
You now have the skills to think like a detective of molecules, piecing together evidence from different “witnesses” — IR, NMR, and Mass Spec — to solve structural mysteries. Afrilearn believes you’re becoming the kind of chemist who can crack even the toughest problems.
