Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my brilliant scientist-in-training! It’s a pleasure to see you back, ready to strengthen your chemistry muscles. Today, we will be looking at oxidation and reduction methods — two of the most important types of reactions in organic chemistry. These are the transformations that change how molecules behave, and they are at the heart of industrial processes, pharmaceuticals, and even everyday life in Nigeria.
Oxidation & Reduction Methods
Meaning of Oxidation and Reduction in Organic Chemistry
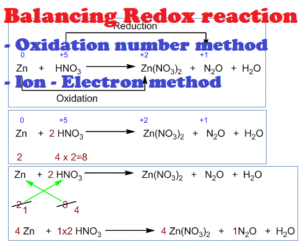
In organic chemistry, oxidation usually means increasing the number of bonds from carbon to oxygen (or other electronegative atoms) or decreasing the number of bonds from carbon to hydrogen. Reduction is the opposite — increasing C–H bonds or decreasing C–O bonds. This is not just about gaining or losing electrons as in inorganic chemistry, but more about changes in bonding patterns.
Common Oxidising Agents
Strong oxidising agents include potassium permanganate (KMnO₄) and chromic acid (H₂CrO₄), which can convert primary alcohols to carboxylic acids and secondary alcohols to ketones. Milder oxidising agents like pyridinium chlorochromate (PCC) stop at the aldehyde stage. In Nigerian chemical industries, KMnO₄ is often used for cleaning and in oxidation processes for fragrances and dyes.
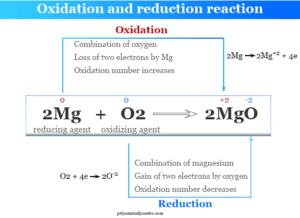
Common Reducing Agents
Metal hydrides like lithium aluminium hydride (LiAlH₄) and sodium borohydride (NaBH₄) are widely used to reduce carbonyl compounds to alcohols. Catalytic hydrogenation, using H₂ gas with a metal catalyst such as palladium or nickel, reduces alkenes and alkynes to alkanes. In the food industry, hydrogenation is used to turn vegetable oils into margarine.
Selective Oxidation and Reduction
Chemists often need to target one functional group without affecting others. For example, PCC can oxidise an alcohol to an aldehyde without touching other sensitive groups. Sodium borohydride can reduce aldehydes and ketones but not esters or carboxylic acids. This selectivity is crucial in multi-step synthesis.
Biological Oxidation and Reduction
In living organisms, oxidation and reduction happen through enzymes like dehydrogenases and oxidases. For instance, in your body, glucose is oxidised in cellular respiration to release energy, and NADH acts as a reducing agent in biosynthetic processes.
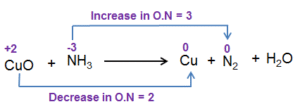
Industrial and Environmental Applications
Industries use oxidation to produce chemicals like acetic acid and phenol. In environmental control, oxidation methods help remove pollutants from water and air. Reduction processes are used to recover metals from ores and to treat harmful nitrogen oxides in vehicle exhaust systems.
If you start with ethanol and treat it with PCC, it will oxidise to ethanal. Using potassium dichromate in acidic medium, ethanal can further oxidise to ethanoic acid. On the reduction side, if you take propanone and treat it with NaBH₄, it reduces to propan-2-ol.
Summary
- Oxidation in organic chemistry increases C–O bonds or decreases C–H bonds, while reduction does the opposite.
- Common oxidising agents include KMnO₄, H₂CrO₄, and PCC, each with different strengths.
- Common reducing agents include LiAlH₄, NaBH₄, and catalytic hydrogenation.
- Selective oxidation/reduction targets specific functional groups without affecting others.
- These reactions have wide applications in biology, industry, and environmental processes.
Evaluation
- Define oxidation and reduction in organic chemistry terms.
- Name two strong oxidising agents and one mild oxidising agent.
- Which reducing agent is strong enough to reduce esters?
- Give one example of a selective oxidation reaction.
- Mention one biological and one industrial example of oxidation or reduction.
You are becoming a master of chemical transformation, understanding how to control reactions to get exactly what you want. Afrilearn is proud to see you gaining skills that are valued in laboratories, industries, and research centres worldwide.
