Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my curious learner! Today, we are stepping into a very elegant area of organic chemistry where reactions happen in a perfectly coordinated way — as if the electrons are dancing in harmony. These are called pericyclic reactions. By the end of this lesson, you will see why these reactions are not only beautiful in theory but also powerful in making complex molecules in a single step.
Pericyclic Reactions I
Meaning of Pericyclic Reactions
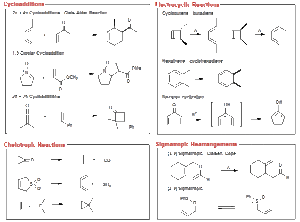
Pericyclic reactions are a class of organic reactions that proceed through a concerted mechanism — all bonds break and form at the same time in a single step, without intermediates. The process involves a cyclic redistribution of bonding electrons.
Key Features of Pericyclic Reactions
They are stereospecific: the stereochemistry of the starting material determines the stereochemistry of the product.
They are concerted: no carbocation, carbanion, or radical intermediates.
They often occur under thermal or photochemical conditions.

Types of Pericyclic Reactions
Cycloaddition reactions: Two or more unsaturated molecules combine to form a cyclic product, e.g., the Diels–Alder reaction between a diene and a dienophile.
Electrocyclic reactions: A single conjugated polyene undergoes ring closure or opening, e.g., butadiene converting to cyclobutene.
Sigmatropic rearrangements: A sigma bond moves across a π-system, with the π-bonds shifting position, e.g., the Claisen rearrangement.
Molecular Orbital Considerations
Pericyclic reactions follow the Woodward–Hoffmann rules, which predict whether a reaction will occur under thermal or photochemical conditions based on the symmetry of the molecular orbitals involved.
Cycloaddition Example – The Diels–Alder Reaction
Reactants: 1,3-butadiene and ethene (dienophile).
Conditions: Usually heat, no catalyst required.
Product: Cyclohexene derivative.
This reaction is important in the synthesis of natural products like steroids and plant fragrances.
Electrocyclic Reaction Example
Under heat, hexatriene can close into cyclohexadiene with a specific stereochemistry dictated by orbital symmetry rules.
Sigmatropic Rearrangement Example
In the Claisen rearrangement, allyl vinyl ethers rearrange upon heating to give γ,δ-unsaturated carbonyl compounds.
Applications in Nigerian Context
Synthesis of fragrance molecules used in local cosmetics and perfumery.
Production of cyclic structures for drug molecules.
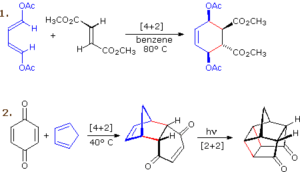
Development of agricultural chemicals with precise molecular architectures.
When cyclopentadiene reacts with maleic anhydride in a Diels–Alder reaction, the product is an endo-norbornene derivative — an important intermediate in polymer and drug synthesis.
Summary
- Pericyclic reactions occur through a concerted cyclic electron movement.
- They include cycloaddition, electrocyclic reactions, and sigmatropic rearrangements.
- They follow stereospecific and orbital symmetry rules predicted by Woodward–Hoffmann principles.
- The Diels–Alder reaction is a classic cycloaddition important in synthesis.
- Nigerian industries can apply these reactions in pharmaceuticals, fragrances, and agrochemicals.
Evaluation
- Define a pericyclic reaction and name its three main types.
- What is the defining feature of a concerted reaction?
- Which reaction type involves a diene and a dienophile?
- State one application of pericyclic reactions in industry.
- What rules help predict whether a pericyclic reaction occurs thermally or photochemically?
You are unlocking a powerful part of organic chemistry that makes the creation of complex molecules elegant and efficient. With Afrilearn, your understanding is preparing you to see chemistry as both a science and an art.
