Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my determined scholar! Today, we continue our journey into the world of pericyclic reactions, but this time, we go deeper into how they work, how to predict their outcomes, and how to use them for creating very specific molecular structures. By the end, you will not only know the theory but also see their real-life value in Nigerian industries and global research.
Pericyclic Reactions II
Thermal vs Photochemical Pericyclic Reactions
Pericyclic reactions can be driven by heat (thermal) or by light (photochemical energy). The energy source determines the orbital symmetry and thus the stereochemical pathway. For example, some reactions that are symmetry-allowed thermally may be forbidden photochemically, and vice versa.
Woodward–Hoffmann Rules in Detail
These rules help predict whether a pericyclic reaction is allowed or forbidden based on:
The number of π-electrons in the reacting system.
Whether the reaction is under thermal or photochemical conditions.
For example, a thermal [4+2] cycloaddition (like Diels–Alder) is allowed, but a thermal [2+2] cycloaddition is forbidden unless light is used.
Advanced Cycloadditions
[2+2] Cycloaddition: Two alkenes combine to form cyclobutanes, usually photochemically.
[6+4] Cycloaddition: Rare but possible in certain natural product syntheses.
Hetero-Diels–Alder Reaction: A heteroatom (like oxygen or nitrogen) is part of the diene or dienophile, leading to heterocyclic products used in pharmaceuticals.
Advanced Electrocyclic Reactions
Thermal Ring Opening of Cyclobutenes: Produces conjugated dienes with specific stereochemistry.
Photochemical Ring Closure: Conjugated trienes can form cyclohexadienes under light with opposite stereochemical outcomes compared to thermal processes.
Advanced Sigmatropic Rearrangements
Cope Rearrangement: A [3,3]-sigmatropic shift in 1,5-dienes that happens under heat, rearranging carbon skeletons.
Claisen Rearrangement Variants: Can involve allyl phenyl ethers to form ortho-allyl phenols, useful in flavour compound synthesis.
Applications in Synthesis
Pharmaceuticals: Building complex drug frameworks like antibiotics or antiviral agents.
Agrochemicals: Creating stable yet active pesticide structures.
Materials Science: Developing light-responsive polymers for smart packaging.
Nigerian Context
In Nigeria, pericyclic reactions could enhance local pharmaceutical production by enabling one-step syntheses of complex molecules, reducing costs and increasing access. They can also help in flavour and fragrance industries, for instance in cocoa and palm oil product enhancement.
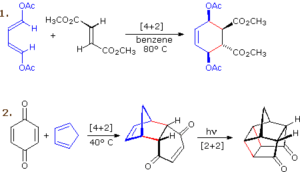
A photochemical [2+2] cycloaddition between two ethene molecules yields cyclobutane, a building block in synthetic organic chemistry. This reaction wouldn’t happen thermally due to orbital symmetry restrictions.
Summary
- Pericyclic reactions can be thermal or photochemical, and their pathway depends on orbital symmetry.
- The Woodward–Hoffmann rules predict which reactions are allowed under given conditions.
- Advanced cycloadditions include [2+2] and hetero-Diels–Alder reactions.
- Electrocyclic and sigmatropic rearrangements can create or modify complex molecular frameworks.
- Applications in Nigeria include pharmaceuticals, agrochemicals, and materials science.
Evaluation
- Explain the difference between thermal and photochemical pericyclic reactions.
- State the basic principle behind the Woodward–Hoffmann rules.
- Give one example of a photochemical pericyclic reaction.
- What is a hetero-Diels–Alder reaction?
- Mention one Nigerian industry that can benefit from pericyclic reaction technology.
You are now mastering reactions that bring creativity and precision together. With Afrilearn, every concept you learn becomes a powerful tool to shape innovations in science, industry, and life itself.
