Back to: Organic Chemistry 300 Level
Welcome to class!
Hello my bright learner! Today, we step into the amazing world of carbohydrates — the sweet and essential biomolecules that power our bodies and give structure to living organisms. From the garri and rice we eat in Nigeria to the glucose that fuels your brain during an exam, carbohydrates are at the heart of life’s energy and structure.
Biomolecules I – Carbohydrate Chemistry
Meaning and Importance of Carbohydrates
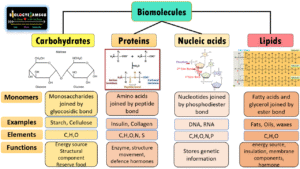
Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen, usually in the ratio Cn(H₂O)n. They are the most abundant biomolecules on Earth and play vital roles in energy storage, structural support, and cellular communication.
Classification of Carbohydrates
Monosaccharides: Single sugar units, e.g., glucose, fructose, galactose.
Disaccharides: Two sugar units linked together, e.g., sucrose (table sugar), lactose (milk sugar), maltose (malt sugar).
Oligosaccharides: Chains of 3–10 monosaccharide units, often attached to proteins and lipids.
Polysaccharides: Long chains of monosaccharide units, e.g., starch, glycogen, cellulose.
Structure of Monosaccharides
Monosaccharides are classified based on:
Number of carbon atoms: trioses (3C), pentoses (5C), hexoses (6C).
Type of carbonyl group: aldoses (aldehyde group) and ketoses (ketone group).
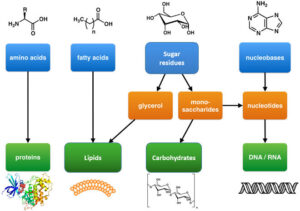
They can exist in straight-chain or ring forms. In aqueous solutions, most monosaccharides form cyclic structures through intramolecular reactions.
Isomerism in Carbohydrates
Structural isomers: Same formula but different connectivity.
Stereoisomers: Same connectivity but different spatial arrangement.
Enantiomers: Mirror-image isomers, e.g., D-glucose and L-glucose.
Epimers: Differ at one carbon atom, e.g., D-glucose and D-galactose.
Reactions of Monosaccharides
Oxidation: Glucose can be oxidised to gluconic acid.
Reduction: Monosaccharides can be reduced to sugar alcohols like sorbitol.
Glycosidic bond formation: Monosaccharides can link via glycosidic bonds to form larger carbohydrates.
Polysaccharides and Their Roles
Starch: Main energy storage in plants; abundant in yam, cassava, and maize.
Glycogen: Animal energy storage; found in liver and muscles.
Cellulose: Structural material in plant cell walls; source of dietary fibre.
Chitin: Structural material in insects and crustaceans.
Biological Importance
Energy source: Glucose provides immediate energy via glycolysis and respiration.
Structural: Cellulose in plant stems, chitin in insect exoskeletons.
Recognition: Oligosaccharides on cell surfaces act in cell recognition and immune responses.
Nigerian Context
In Nigeria, carbohydrate-rich foods like pounded yam, eba, rice, and plantain are major dietary staples. Understanding carbohydrate chemistry helps in food technology, diabetes research, and industrial fermentation (e.g., bread and beer production).
When glucose and fructose join via a glycosidic bond, they form sucrose — the common sugar used in tea. Hydrolysing sucrose with an acid or enzyme breaks it back into glucose and fructose, ready for metabolism.
Summary
- Carbohydrates are carbon, hydrogen, and oxygen compounds vital for energy, structure, and cell signalling.
- They are classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides.
- Monosaccharides can exist as straight-chain or cyclic structures and show various isomeric forms.
- Common reactions include oxidation, reduction, and glycosidic bond formation.
- Polysaccharides like starch, glycogen, and cellulose have distinct storage and structural roles.
Evaluation
- Define carbohydrates and give two examples.
- State four classes of carbohydrates with one example each.
- Distinguish between aldoses and ketoses.
- What is a glycosidic bond?
- Name two polysaccharides and their functions.
Every time you eat your favourite carbohydrate-rich food, you’re engaging with nature’s energy currency. With Afrilearn, you are not just learning facts — you are learning how life itself is powered.
