Back to: Organic Chemistry 300 Level
Welcome to class!
Hello, my sharp-minded learner! Today, we’re moving deeper into the fascinating world of aromatic substitution, focusing on more advanced ideas. You’ve already learnt the basics of electrophilic aromatic substitution (EAS), but in this lesson, we’ll tackle factors that control how and where substitution happens, how multiple substituents interact, and even some special aromatic substitution reactions. Think of this as understanding why certain spices in a Nigerian soup change the whole taste — one small addition can direct everything else!
Aromatic Substitution Advanced
Recap of Aromatic Substitution
Aromatic substitution replaces a hydrogen atom on an aromatic ring with another group while keeping the aromatic stability intact. In EAS, an electrophile attacks the ring to form a sigma complex, followed by deprotonation to restore aromaticity.
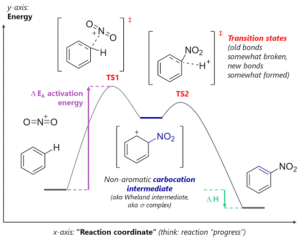
Substituent Effects on Reactivity
Substituents already present on the ring can make it more or less reactive towards further substitution.
Activating groups increase reactivity (e.g., -OH, -NH₂, alkyl groups). They donate electron density to the ring, making it more attractive to electrophiles.
Deactivating groups decrease reactivity (e.g., -NO₂, -CF₃, -SO₃H). They withdraw electron density, making the ring less reactive.
Substituent Effects on Orientation
Substituents also determine where the new group will attach:
Ortho/para-directing groups: Usually activating, e.g., -OH, -OCH₃, -NH₂.
Meta-directing groups: Usually deactivating, e.g., -NO₂, -CN, -SO₃H.
Multiple Substituent Effects
When two or more substituents are on the ring:
The stronger activating group generally controls the orientation of the new substitution.
If substituents are in positions that conflict, the major product depends on the balance between steric hindrance and electronic effects.
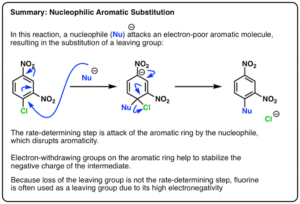
Special Types of Aromatic Substitution
Nucleophilic Aromatic Substitution (NAS): Occurs when an electron-withdrawing group activates the ring for attack by a nucleophile. Common in nitro-substituted aromatic halides.
Benzyne Mechanism: A high-energy pathway for NAS involving elimination–addition.
Aromatic Substitution via Radical Mechanisms: For example, halogenation under light and heat.
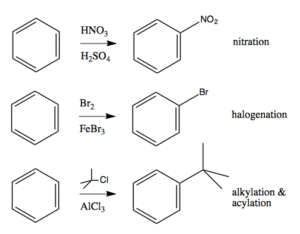
Industrial and Biological Importance
Aromatic substitution is key in making dyes, perfumes, medicines, and pesticides.
In Nigeria’s pharmaceutical sector, the synthesis of aspirin, paracetamol, and antimalarial drugs relies heavily on aromatic substitution chemistry.
The production of flavouring agents like vanillin from aromatic precursors involves selective substitution steps to install functional groups at the right positions without destroying the aromatic ring.
Bromination of anisole (methoxybenzene) with Br₂ in FeBr₃ produces mainly para-bromoanisole because the -OCH₃ group is strongly activating and directs to the ortho and para positions, with the para product dominating due to less steric hindrance.
Summary
- Aromatic substitution retains aromaticity while replacing a hydrogen atom on the ring.
- Substituents affect both reactivity and orientation of new substituents.
- Activating groups are usually ortho/para directors, while deactivating groups are often meta directors.
- Multiple substituents interact, with the stronger activating group usually controlling orientation.
- Advanced forms include nucleophilic aromatic substitution, benzyne mechanisms, and radical substitution.
Evaluation
- Distinguish between activating and deactivating groups with examples.
- Explain the difference between ortho/para and meta directors.
- Describe what happens when two substituents direct to different positions.
- State two special types of aromatic substitution and give examples.
- Why is bromination of anisole mainly para-directed?
You are now mastering the deeper logic behind aromatic substitution — the same logic that drives countless chemical processes in industry and nature. Keep this momentum, and every complex reaction will start making perfect sense with Afrilearn.
