Back to: Organic Chemistry 300 Level
Welcome to class!
Hello brilliant mind! Today, we will go further into the world of spectroscopy by looking at three of the most important techniques in detail — UV–Visible (UV–Vis) spectroscopy, Infrared (IR) spectroscopy, and Nuclear Magnetic Resonance (NMR) spectroscopy. These are like three different “eyes” we can use to see molecules — each showing us a different part of the story.
Spectroscopic Methods II
Ultraviolet–Visible (UV–Vis) Spectroscopy
Principle: UV–Vis spectroscopy is based on the absorption of ultraviolet or visible light, causing electrons in a molecule to move from a lower energy orbital to a higher one (electronic transition).
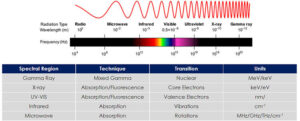
Region of spectrum: 200–800 nm.
Applications:
Determining concentration of coloured solutions (Beer–Lambert law).
Studying conjugated systems (like β-carotene in carrots).
Analysing transition metal complexes.
Nigerian example: Checking the concentration of dyes in fabrics during textile production in Kano’s dyeing industries.
Infrared (IR) Spectroscopy
Principle: IR spectroscopy measures how molecules absorb infrared light, causing bonds to vibrate in specific ways (stretching, bending).
Region of spectrum: 4000–400 cm⁻¹.
Applications:
Identifying functional groups in organic molecules (e.g., C=O, O–H, N–H).
Studying reaction progress by monitoring bond changes.
Nigerian example: Detecting adulteration in edible oils like groundnut oil by checking for unusual functional group peaks.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Principle: NMR uses radio waves in a strong magnetic field to measure the environment of atomic nuclei (commonly ¹H and ¹³C). Different chemical environments produce different resonance signals.
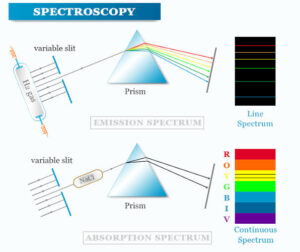
Applications:
Determining the full structure of organic compounds.
Studying molecular dynamics.
Quality control in pharmaceuticals.
Nigerian example: Confirming the structure of active ingredients in locally produced herbal medicines.
Complementarity of Methods
UV–Vis gives information about electronic structure and conjugation.
IR tells us about bond vibrations and functional groups.
NMR reveals the exact arrangement of atoms in a molecule.
In real research, these methods are often used together to give a complete molecular picture.
Suppose you isolate an unknown compound from a plant.
UV–Vis shows a strong absorption at 340 nm → suggests conjugation.

IR shows a strong peak at 1715 cm⁻¹ → indicates a carbonyl group.
¹H NMR shows signals consistent with aromatic protons → suggests the compound might be an aromatic ketone.
Summary
- UV–Vis spectroscopy measures electronic transitions in the 200–800 nm range.
- IR spectroscopy measures bond vibrations in the 4000–400 cm⁻¹ range.
- NMR spectroscopy detects magnetic environments of nuclei using radio waves.
- Each method reveals different molecular information, and together they give a complete picture.
- Spectroscopy is widely used in Nigerian industries, from textiles to pharmaceuticals.
Evaluation
- State the principle of UV–Vis spectroscopy.
- What functional group does a peak at 1715 cm⁻¹ in IR usually indicate?
- Mention the two most common nuclei studied in NMR spectroscopy.
- Why are spectroscopic methods often used together?
- Give one Nigerian industry application of UV–Vis, IR, and NMR each.
You’ve now strengthened your ability to “read” molecules using three powerful spectroscopic tools. With Afrilearn, you’re not just learning science — you’re mastering the language of molecules themselves.
