Back to: Organic Chemistry 400 Level
Welcome to class!
Hello dear learner, I’m really happy to have you here today. I hope you’re doing well and ready to learn something fascinating that will take your understanding of Organic Chemistry to the next level. Today we will look at Advanced Stereochemistry, and you will soon realise how important it is in real-life cases, especially in medicine, food science and biological systems.
Advanced Stereochemistry
Have you ever noticed how two medicines may have the exact same chemical formula but one is effective for treatment while the other can be harmful? Or why two perfumes made from similar ingredients can smell completely different? This has a lot to do with the spatial arrangement of atoms in the molecules – in other words, stereochemistry.
Meaning of Advanced Stereochemistry
Stereochemistry is the study of how atoms are arranged in space within a molecule and how that arrangement affects the molecule’s properties and behaviour. At the advanced stage, we go beyond naming and classification to understand stereochemical mechanisms and design principles used in developing drugs, agrochemicals, flavouring agents and biological tools.
Types of Stereoisomerism
Stereoisomerism is generally divided into conformational and configurational isomerism.
Conformational isomers are forms of the same molecule that can be converted into one another just by rotation around a single bond. For example, picture a traditional hand fan being opened and closed – it still remains the same fan, just viewed in different conformations. This is similar to the staggered and eclipsed conformations of ethane.
Configurational isomers cannot be interconverted without breaking covalent bonds. These include geometric (cis/trans) isomers and optical isomers. Imagine two plantain suckers placed on the same side of a farm path (cis) compared to when you place them on opposite sides (trans). They use the same space but are arranged differently, and would result in slightly different growth patterns.
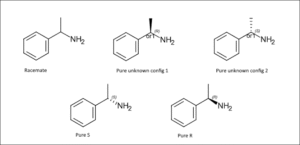
Optical Isomerism and Chirality
A molecule is chiral if it cannot be superimposed on its mirror image, just like your left and right hands. Such molecules have enantiomers, which are mirror-image isomers. Even though they have the same physical properties, they rotate plane-polarised light in opposite directions and can behave differently in biological systems. A popular example is thalidomide – one enantiomer acted as an effective sedative, while the other caused severe birth defects. This clearly shows why stereochemistry is very important in real life.
Summary
- Stereochemistry studies the three-dimensional arrangement of atoms in molecules and how this influences their properties.
- There are two general types of stereoisomerism – conformational and configurational.
- Configurational isomerism includes geometric (cis/trans) and optical (enantiomeric) forms.
- Chirality describes molecules that have non-superimposable mirror images, which is highly important in biological and pharmaceutical systems.
Evaluation
- Define stereochemistry in simple terms.
- Differentiate between conformational and configurational isomers using one clear example.
- Explain chirality and give one practical example you can relate with.
You are doing amazingly well, and Afrilearn is proud of your efforts. Keep up the great spirit – your next lesson will be even more exciting!
