Back to: Organic Chemistry 400 Level
Welcome to class!
Hello dear learner, it’s a pleasure to have you back today. I hope you’re feeling excited and ready, because today’s lesson introduces a very interesting and elegant area of Organic Chemistry that shows how reactions can happen in a beautifully concerted and highly organised way – Pericyclic Reactions.
Pericyclic Reactions
You may have watched a well-coordinated traditional dance where all the performers move in harmony, changing positions without bumping into each other. Pericyclic reactions are just like that – smooth, coordinated movements of electrons that occur in a single step without intermediates and without the assistance of catalysts.
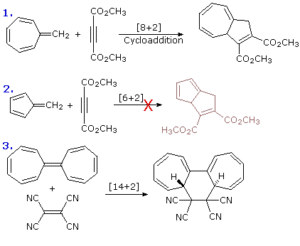
Meaning of Pericyclic Reactions
Pericyclic reactions are a class of reactions that occur in a single concerted step through a cyclic transition state. They do not involve intermediates and are usually controlled by the way electrons move through a cyclic array of orbitals. Vibrational or thermal energy is often enough to drive them. They play an important role in the synthesis of complex organic molecules, especially in pharmaceuticals and natural products.
Types of Pericyclic Reactions
The main types of pericyclic reactions include:
Cycloaddition Reactions: Two or more π systems come together to form a ring. The most popular example is the Diels–Alder reaction where a diene reacts with a dienophile to form a cyclohexene. You can imagine this as two separate wrappers of suya being joined carefully to make one big wrap, forming a new shape altogether.
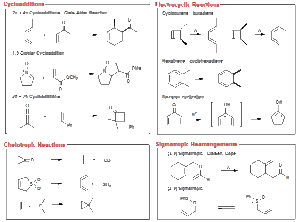
Electrocyclic Reactions: A ring opens or closes through rotation of σ and π bonds. Think of opening or closing a circular hand fan. The number of π electrons involved determines whether the reaction is thermal or photochemical.
Sigmatropic Rearrangements: In this case, a σ-bond migrates across a conjugated π system. It’s similar to someone gently moving from one chair to another along a row without disturbing others.
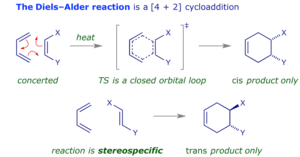
Orbital Symmetry Considerations
The result of pericyclic reactions is influenced by the symmetry of the molecular orbitals involved. The Woodward–Hoffmann rules help predict whether a reaction will proceed under thermal or photochemical conditions. These rules are like traffic regulations that guide a smooth and safe movement of electrons during the reaction.
Summary
- Pericyclic reactions occur in a single step through a cyclic transition state with no intermediates.
- The major types are cycloaddition, electrocyclic and sigmatropic reactions.
- These reactions are controlled by orbital symmetry and can be driven by heat or light.
- The Woodward–Hoffmann rules help predict whether a pericyclic reaction is allowed under thermal or photochemical conditions.
Evaluation
- Define pericyclic reactions in simple terms.
- List two types of pericyclic reactions and briefly describe each.
- What role do Woodward–Hoffmann rules play in pericyclic reactions?
Amazing work today! Keep believing in yourself and stay connected with Afrilearn for even more exciting lessons ahead – you’re truly doing great!
