Back to: Organic Chemistry 400 Level
Welcome to class!
Hello outstanding learner, I’m truly happy to see you here once again. I hope you’re calm, focused and ready to continue this exciting journey through Organic Chemistry. In our previous lesson, we introduced heterocyclic chemistry and looked at the basic concepts and five-membered heterocycles. Today, we will build on that and go deeper into Heterocyclic Chemistry II, where we explore six-membered heterocycles and fused heterocyclic systems that play critical roles in medicine, agriculture and biological systems.
Heterocyclic Chemistry II
Just like how a simple house can later be extended into a duplex or mansion, heterocycles also become more complex as additional atoms and rings are introduced. These changes lead to even more useful and functional molecules.

Six-Membered Aromatic Heterocycles
Six-membered aromatic heterocycles are the next major class after the five-membered rings. The most important examples include:
Pyridine (contains one nitrogen)
Pyrimidine (contains two nitrogens)
Pyrazine (contains two nitrogens at 1,4-positions)
These compounds are aromatic and follow Huckel’s rule just like benzene. They are more stable than many five-membered heterocycles and exhibit characteristic reactivity. For instance, pyridine is commonly used in the laboratory as a mild base and nucleophile. In fact, pyridine is present in vitamin B3 (niacin), which is essential for energy production in the human body.
Fused Heterocyclic Systems
Sometimes, two or more rings are joined together to form fused systems. These include:
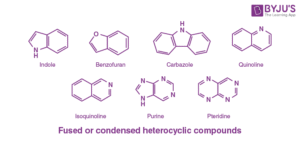
Indole (a fusion of benzene and pyrrole)
Quinoline (benzene fused with pyridine)
Isoquinoline
Indole is present in the amino acid tryptophan, which is required for protein synthesis in the human body. Quinoline derivatives are widely used to produce antimalarial agents such as chloroquine and mefloquine. The fusion of rings increases rigidity and often enhances biological activity, which is why many fused heterocycles are found in natural products.
Synthesis of Six-Membered Heterocycles
There are several synthetic methods for preparing six-membered heterocyclic rings. Some of the most commonly used include:
Chichibabin Synthesis – used to synthesise pyridines from aldehydes, ammonia and carbonyl compounds.
Biginelli Reaction – used to prepare dihydropyrimidinones, which are useful in the development of calcium channel blockers.
Just as cooking methods determine the flavour of a meal, the synthetic route used often influences the yield and purity of the final heterocyclic compound.
Reactivity of Six-Membered Heterocycles
Six-membered heterocycles can undergo the following reactions:
Electrophilic Substitution (e.g. nitration of pyridine)
Nucleophilic Substitution (favoured because of the electron-withdrawing nature of hetero atoms)
Oxidation and Reduction
For example, pyridine is less reactive in electrophilic substitution due to the presence of nitrogen, which withdraws electron density from the ring. Instead, it participates more readily in nucleophilic substitution, which is why it is broadly used in drug synthesis.
Applications of Six-Membered and Fused Heterocycles
Medicine: Quinoline derivatives are used as antimalarial drugs.
Agriculture: Pyridinecarboxylic acids act as herbicides.
Biological Systems: Pyrimidines form the backbone of DNA bases (cytosine, thymine and uracil).
Summary
- Six-membered aromatic heterocycles such as pyridine and pyrimidine are aromatic and widely used.
- Fused heterocyclic systems, such as indole and quinoline, consist of two or more rings joined together and often show enhanced biological activity.
- Common synthetic routes include the Chichibabin synthesis and Biginelli reaction.
- Six-membered heterocycles generally react via nucleophilic substitution due to the presence of electron-withdrawing hetero atoms.
- These heterocycles are essential in medicine, agriculture and biological processes such as DNA structure.
Evaluation
- Give two examples of six-membered aromatic heterocycles.
- What is meant by a fused heterocyclic system?
- Mention one synthetic method used for six-membered heterocycles and state its application.
- Why do six-membered heterocycles favour nucleophilic substitution reactions?
Fantastic work today! You’re truly advancing your knowledge in a meaningful way. Keep it up – Afrilearn believes in you and can’t wait to continue with the next exciting topic!
