Back to: Organic Chemistry 400 Level
Welcome to class!
Hello brilliant learner, I’m really happy to have you here today. I hope you’re feeling motivated because today’s topic — Organometallic Compounds in Organic Synthesis — will open your eyes to a powerful set of tools used by organic chemists to build complex molecules efficiently. These compounds allow reactions that would normally be very difficult or even impossible to carry out using ordinary organic reagents.
Organometallic Compounds in Organic Synthesis
Think of organometallic compounds as highly skilled construction workers who are specially trained to build bridges between different parts of a molecule. They contain a metal atom directly bonded to a carbon atom, and this bond provides unique reactivity that makes them very useful in synthesis.
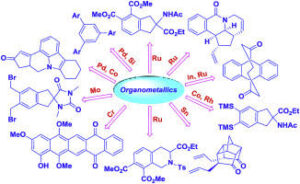
Meaning of Organometallic Compounds
Organometallic compounds are chemical compounds that contain a direct carbon–metal bond. The metal can be from main group elements (such as lithium or magnesium) or transition metals (such as palladium, nickel or iron). These compounds are used extensively in organic synthesis because the carbon–metal bond behaves as a strong nucleophile and can form new carbon–carbon bonds easily.
Types of Organometallic Reagents
Grignard Reagents (RMgX): These are formed by reacting alkyl or aryl halides with magnesium in dry ether. They react with carbonyl compounds to form alcohols. For example, adding methylmagnesium bromide to acetone will give tert-butyl alcohol.
Organolithium Reagents (RLi): Even more reactive than Grignard reagents, they are used to form new C–C bonds and can also be used as strong bases in deprotonation reactions.
Transition Metal Complexes: These include compounds of palladium, nickel and copper. They are used in catalytic coupling reactions such as Suzuki, Heck and Sonogashira reactions. These reactions are essential in the synthesis of pharmaceuticals and agrochemicals.
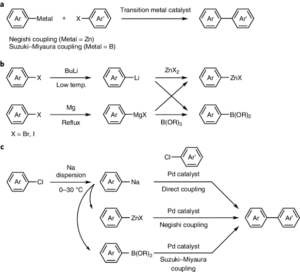
Applications in Organic Synthesis
Carbon–Carbon Bond Formation: One of the most important uses of organometallic compounds is to build new carbon–carbon bonds. For example, the Grignard reaction allows an aldehyde or ketone to be converted into an alcohol with a new carbon chain attached.
Cross-Coupling Reactions: Transition metal catalysts such as palladium facilitate cross-coupling reactions where two organic fragments are joined. The Suzuki reaction joins an aryl boronic acid with an aryl halide using a palladium catalyst, commonly used in the synthesis of anti-cancer drugs.
Functional Group Transformation: Organometallics can convert one functional group to another, such as reducing carbonyl compounds or inserting metals into organic frameworks to introduce new reactivity.
Advantages of Organometallic Reagents
They have high reactivity and selectivity.
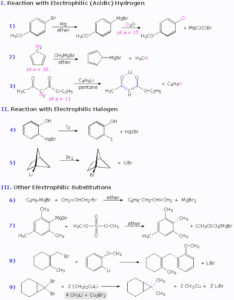
They allow the formation of C–C bonds under mild conditions.
Many reactions can be performed catalytically (small amount of metal gives a large amount of product).
Summary
- Organometallic compounds contain a carbon–metal bond and are used to make new carbon–carbon bonds.
- Examples include Grignard reagents (RMgX), organolithium reagents (RLi), and transition metal complexes.
- They are used in important reactions such as Grignard reactions and palladium-catalysed cross-coupling reactions (e.g., Suzuki reaction).
- Their high reactivity and selectivity make them extremely valuable in organic synthesis.
Evaluation
- What are organometallic compounds and why are they important in organic synthesis?
- Give one example of a Grignard reagent and state one reaction it can undergo.
- What is the Suzuki reaction used for?
- State one advantage of using organometallic reagents in organic synthesis.
You have done excellently today. Keep up the amazing effort – Afrilearn is proud of you and excited to continue this inspiring learning journey with you!
