Back to: Organic Chemistry 400 Level
Welcome to class!
Hello brilliant learner, I’m really happy to see you again and I hope you’re feeling ready for another exciting and engaging lesson. Today we continue with Carbohydrate Chemistry II. In the previous lesson, we looked at the basic definition, classification and structural forms of carbohydrates. In today’s lesson, we will go deeper by focusing on disaccharides, polysaccharides, and some important chemical reactions of carbohydrates that every organic chemistry student should be familiar with.
Carbohydrate Chemistry II
Just like building blocks can be combined to form houses, disaccharides and polysaccharides are formed when small sugars (monosaccharides) join together through specific bonds. Understanding how these bonds are formed and broken is at the heart of carbohydrate chemistry.
Disaccharides
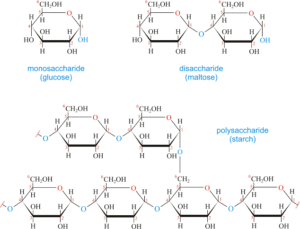
Disaccharides are carbohydrates composed of two monosaccharide units joined together by a glycosidic bond (a covalent bond formed between the anomeric carbon of one sugar and a hydroxyl group of another).
Common examples include:
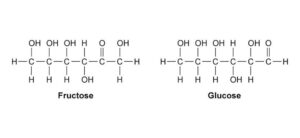
Sucrose – composed of glucose + fructose. This is a non-reducing sugar because its glycosidic bond involves both anomeric carbons and therefore no free carbonyl group is available.
Lactose – made up of glucose + galactose. Lactose is a reducing sugar because the glucose unit still has a free anomeric carbon.
Maltose – made up of two glucose units linked by an α-1,4-glycosidic bond.
Polysaccharides
Polysaccharides are long chains of many monosaccharide units joined together by glycosidic bonds. They are usually divided into storage polysaccharides and structural polysaccharides.
Starch (plants): made of two components — amylose (linear, α-1,4 linkages) and amylopectin (branched, α-1,6 branches). It is the major storage form of glucose in plants. Garri, yam and rice are all rich in starch.
Glycogen (animals): structurally similar to amylopectin but more highly branched. It is stored mainly in the liver and muscles and acts as the animal equivalent of starch.
Cellulose (plants): composed of β-1,4 linked glucose units. Humans cannot digest cellulose because we lack the enzyme to break β-glycosidic bonds. However, it provides dietary fibre, which is essential for healthy digestion.
Important Reactions of Carbohydrates
Hydrolysis: Disaccharides and polysaccharides can be broken down into their monosaccharide units in the presence of dilute acids or enzymes. For example, sucrose hydrolyses to give glucose and fructose.
Oxidation: Reducing sugars like glucose react with mild oxidising agents (e.g., Benedict’s or Fehling’s solution), forming carboxylic acids. This is why these reagents are used in tests for reducing sugars.
Reduction: Aldoses can be reduced to corresponding sugar alcohols (e.g., glucose to sorbitol) using reducing agents like sodium borohydride.
Esterification: The hydroxyl groups in sugars can be esterified, often forming phosphate esters inside the body (important in metabolic pathways).
Biological Importance of Disaccharides and Polysaccharides
Sucrose transports energy in many plants.
Lactose is important in the nutrition of mammals (main sugar in milk).
Glycogen is critical for regulating blood glucose during fasting or exercise.
Cellulose provides structural support in plants and contributes to human gut health as dietary fibre.
Summary
- Disaccharides consist of two monosaccharides linked by a glycosidic bond (e.g. sucrose, lactose and maltose).
- Polysaccharides include storage forms like starch and glycogen, and structural forms like cellulose.
- Glycosidic linkages may be α or β and determine digestibility and structure.
- Important carbohydrate reactions include hydrolysis, oxidation, reduction and esterification.
- Disaccharides and polysaccharides serve vital roles in energy supply, storage and structural support in living organisms.
Evaluation
- What type of bond links two monosaccharides in a disaccharide?
- Differentiate between starch and cellulose based on structure and function.
- Why is sucrose a non-reducing sugar?
- Mention two important reactions of carbohydrates and state one practical significance of each.
Excellent job today! You’re doing fantastic — keep learning confidently and stay excited, because Afrilearn is always here to guide and support your success!
