Back to: Organic Chemistry 400 Level
Welcome to class!
Hello exceptional learner, I’m very happy to have you here today. I hope you’re feeling relaxed and ready because today’s topic — Lipid Chemistry — will help you understand another important group of biomolecules found in your food and your body. Just as carbohydrates give us energy quickly and proteins help build the body, lipids play their own unique and essential roles in living organisms.
Lipid Chemistry
If you’ve ever fried plantain with vegetable oil or noticed how body creams keep your skin from drying out, you’ve already experienced the real-life applications of lipids. These molecules are all around us and are vital for healthy living.
Meaning and Classification of Lipids
Lipids are a group of naturally occurring organic compounds that are generally insoluble in water but soluble in organic solvents like chloroform or ether. They mainly consist of carbon, hydrogen and oxygen. Their hydrophobic nature makes them important components of biological membranes and energy storage materials.
Lipids are broadly classified into:
Simple Lipids – such as fats and oils (triglycerides) formed from glycerol and fatty acids.
Compound Lipids – such as phospholipids and glycolipids which contain additional groups like phosphate or carbohydrate.
Derived Lipids – substances derived from simple or compound lipids such as fatty acids, steroids (e.g., cholesterol) and fat-soluble vitamins.
Fats and Oils (Triglycerides)
Triglycerides are composed of one molecule of glycerol joined to three fatty acids by ester bonds.
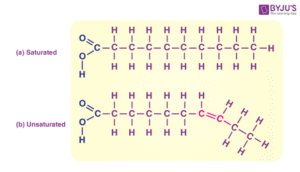
Fats are solid at room temperature (e.g., animal fat, butter).
Oils are liquid at room temperature (e.g., palm oil, olive oil).
The difference lies mainly in the type of fatty acids present — saturated fatty acids form solids, while unsaturated fatty acids form liquids.
For example, shea butter has more saturated fatty acids and is solid, whereas groundnut oil contains more unsaturated fats and remains liquid.
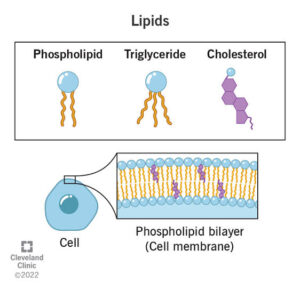
Phospholipids
Phospholipids contain two fatty acids and one phosphate group attached to glycerol. Because they have both hydrophobic and hydrophilic parts, they form cell membranes. Imagine a soap bubble with its surface facing water and the interior protected — that is how phospholipids behave in biological systems.
Functions of Lipids
Energy Storage – Lipids provide more than twice the energy supplied by carbohydrates per gram.
Structural Role – Phospholipids are essential components of cell membranes.
Protection and Insulation – Fat under the skin protects organs and conserves body heat.
Hormone Precursors – Steroids like cholesterol are precursors of hormones such as oestrogen and testosterone.
Fat-Soluble Vitamins – Vitamins A, D, E and K require lipids for absorption.
Summary
- Lipids are hydrophobic organic molecules that include fats, oils, phospholipids, steroids and fatty acids.
- They are classified into simple lipids (e.g., triglycerides), compound lipids (e.g., phospholipids) and derived lipids (e.g., cholesterol).
- Triglycerides are made of glycerol and three fatty acids and serve as major energy storage molecules.
- Phospholipids contain fatty acids and a phosphate group and form the structure of biological membranes.
- Lipids perform essential functions such as energy storage, protection, insulation and hormone production.
Evaluation
- What are lipids and why are they insoluble in water?
- Differentiate between simple and compound lipids with one example each.
- What is the structural difference between fats and oils?
- State two important functions of lipids in living organisms.
Excellent work today! You are building a strong understanding of biomolecules and Afrilearn is very proud of you. Keep going confidently — your next lesson will take you even further in your learning journey!
