Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s wonderful to have you here today. I hope you’re ready to learn something that will help you see the world around you in a fresh way. Imagine for a moment: when you drink a cold sachet of pure water, what makes up that water? Or when you smell fried plantain in the kitchen, what gives off that sweet aroma? Behind all these everyday experiences are atoms, molecules, and ions. Let’s break it down together.
Basic Concepts Of Atoms, Molecules, Ions
Atoms
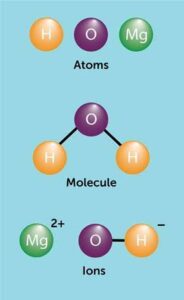
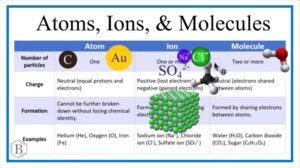
An atom is the smallest particle of an element that can take part in a chemical reaction. Think of an atom like one single grain of rice in a full bag. On its own, it seems small, but put many together and you get something significant. Atoms consist of three main parts: protons and neutrons in the nucleus (centre) and electrons moving around in regions outside the nucleus. For example, the element oxygen is made up of oxygen atoms.
Molecules
Sometimes, atoms do not like to stay alone. They combine with other atoms to form molecules. A molecule is simply two or more atoms chemically bonded together. For instance, when two hydrogen atoms join with one oxygen atom, they form water (H₂O). This is the molecule you drink every day. Another example is oxygen gas (O₂), which we breathe to stay alive. Molecules can be made of the same type of atoms (like O₂) or different atoms (like H₂O).
Ions
Now, atoms can also gain or lose electrons, and when this happens, they become charged particles called ions. If an atom loses electrons, it becomes positively charged and is called a cation. Sodium (Na⁺), found in table salt, is a good example. If an atom gains electrons, it becomes negatively charged and is called an anion, like chloride (Cl⁻). Just like how Nigerians love pairing jollof rice with fried plantain, positive and negative ions often come together to form compounds such as sodium chloride (NaCl), which is common table salt.
Summary
- Atoms: the smallest particles of elements, like grains of rice.
- Molecules: two or more atoms bonded together, like H₂O and O₂.
- Ions: charged atoms formed when electrons are gained or lost (Na⁺, Cl⁻).
Evaluation
- Define an atom in simple terms.
- Give two examples of molecules and state whether their atoms are the same or different.
- Differentiate between a cation and an anion with examples.
You’re doing wonderfully well! Remember, Chemistry is not just about symbols and equations—it’s about understanding the tiny building blocks of everything you see, touch, and use daily. Keep your curiosity alive, and know that Afrilearn is always here to guide you as you grow in knowledge.
