Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m so glad you joined today. Let me start with a simple picture: imagine fetching water into several buckets of different sizes. Naturally, you will fill the smallest bucket first before the bigger ones. In the same way, electrons fill atomic orbitals starting from the lowest energy level before moving to higher ones. This simple but powerful rule is called the Aufbau Principle.
Aufbau Principle
What is the Aufbau Principle?
The word “Aufbau” comes from a German term that means building up. The Aufbau Principle states that electrons enter orbitals in order of increasing energy, starting from the lowest-energy orbital and moving step by step to higher ones. It’s like building a house: you must lay the foundation before adding the walls and roof.
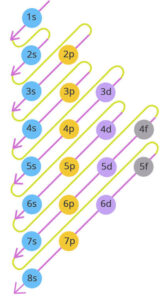
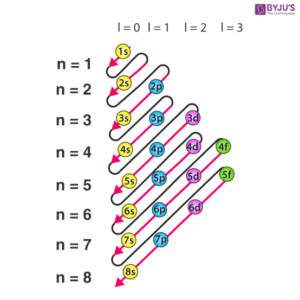
Order of Filling Orbitals
Electrons don’t just fill orbitals randomly. They follow a definite sequence that can be remembered using a diagonal rule or energy level chart. The order is:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p
For example:
Hydrogen (Z = 1): 1s¹
Carbon (Z = 6): 1s² 2s² 2p²
Calcium (Z = 20): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Exceptions to the Rule
Like in life, there are exceptions! Some atoms prefer to rearrange their electrons slightly to achieve greater stability. For example:
Chromium (Z = 24): instead of 1s² … 3d⁴ 4s², it is 1s² … 3d⁵ 4s¹ (because half-filled d-orbitals are more stable).
Copper (Z = 29): instead of 1s² … 3d⁹ 4s², it is 1s² … 3d¹⁰ 4s¹ (because fully filled d-orbitals are more stable).
Why is the Aufbau Principle Important?
This principle helps us:
Predict the electronic configurations of elements.
Understand chemical behaviour of elements.
Explain why elements in the same group behave similarly (since their outermost electron arrangements follow this filling order).
Think of it this way: the Aufbau Principle is like the “exam seating arrangement” for electrons. Everyone must follow the order, no skipping!
Summary
- Aufbau Principle: electrons fill orbitals from the lowest to the highest energy level.
- Order of filling is given by the diagonal rule (1s → 2s → 2p → 3s …).
- Exceptions exist (e.g., chromium and copper).
- It explains the structure of the periodic table and chemical reactivity.
Evaluation
- State the Aufbau Principle in your own words.
- Write the electronic configuration of nitrogen (Z = 7) using the Aufbau Principle.
- Why is copper’s configuration an exception to the Aufbau rule?
Well done, champ! You’ve just mastered one of the golden rules of atomic structure. Remember, every time you see an element’s configuration, it’s the Aufbau Principle at work. Keep shining, and let Afrilearn keep being your learning partner in this exciting journey.
