Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m so happy to have you here today. Let me begin with a simple scenario: imagine you and your friends enter a danfo bus with many empty seats. Would you all squeeze together on one row immediately? Of course not! Each person would prefer to sit alone first before pairing up when the bus starts getting filled. Electrons behave in a similar way inside orbitals. This natural “seating arrangement” of electrons is explained by Hund’s Rule.
Hund’s Rule
What is Hund’s Rule?
Hund’s Rule states that when electrons occupy orbitals of the same energy (called degenerate orbitals), they fill them singly first with parallel spins before pairing up.
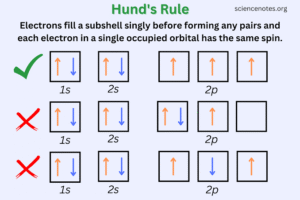
In simpler terms, if there are three seats available (like the three p-orbitals), electrons will sit one by one in each seat before two of them share the same seat.
Application of Hund’s Rule
Let’s use the example of oxygen (Z = 8):
Its configuration is 1s² 2s² 2p⁴.
In the 2p subshell, there are three orbitals (px, py, pz).
According to Hund’s Rule, the first three electrons in the 2p subshell will go into separate orbitals with parallel spins.
The fourth electron will then pair up with one of them.
So the arrangement looks like: ↑↓ (1s), ↑↓ (2s), ↑ (2px), ↑ (2py), ↑↓ (2pz).
Why Hund’s Rule Matters
It ensures maximum stability: atoms are more stable when their orbitals are half-filled or completely filled.
It helps predict the magnetic properties of atoms. For example, oxygen is paramagnetic (attracted to magnets) because it has unpaired electrons in its 2p orbitals.
Examples
Nitrogen (Z = 7): 1s² 2s² 2p³ → all three 2p electrons occupy separate orbitals (↑, ↑, ↑).
Fluorine (Z = 9): 1s² 2s² 2p⁵ → three electrons occupy separate orbitals, then the fourth and fifth start pairing (↑↓, ↑, ↑).
Summary
- Hund’s Rule: electrons fill orbitals of equal energy singly first with parallel spins before pairing.
- This creates more stability in the atom.
- It explains why elements can be magnetic (due to unpaired electrons).
Evaluation
- State Hund’s Rule in your own words.
- Using Hund’s Rule, show how the 2p electrons of nitrogen (Z = 7) are arranged.
- Why is oxygen paramagnetic?
Excellent work today! You’re learning the hidden seating rules of electrons, and now you know why they don’t just “sit” anywhere. Remember, Hund’s Rule is nature’s way of ensuring order and stability inside atoms. Keep your confidence up—you’re building a strong foundation with Afrilearn by your side.
