Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always wonderful to have you here. Let me start with something familiar: think of an exam hall at UNILAG or UI. Each student must have a unique seat number—no two students are allowed to sit in the exact same seat at the same time. That’s the rule. In the atomic world, electrons also follow a strict rule about their “seating arrangement.” This important rule is called the Pauli Exclusion Principle.
Pauli Exclusion Principle
What is the Pauli Exclusion Principle?
Proposed by Wolfgang Pauli in 1925, the Pauli Exclusion Principle states that:
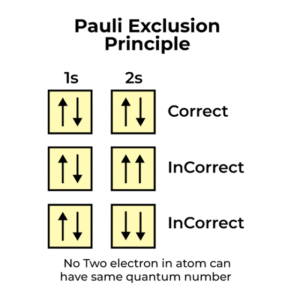
No two electrons in the same atom can have the same set of four quantum numbers.
This simply means that if two electrons are in the same orbital, they must be distinguished by at least one property—in practice, their spins must be opposite.
How It Works
Each orbital can hold a maximum of two electrons. But these two electrons must spin in opposite directions—one spins “up” (+½) and the other spins “down” (–½).
Think of it like a two-seater bench in a lecture hall: only two students can sit there, and one must face left while the other faces right so they don’t clash.
Examples
Hydrogen (Z = 1): 1s¹ → one electron in the 1s orbital.
Helium (Z = 2): 1s² → two electrons in the 1s orbital, but with opposite spins.
Oxygen (Z = 8): 1s² 2s² 2p⁴ → in the 2p orbitals, some electrons pair up with opposite spins, while others remain single.
Importance of the Pauli Exclusion Principle
It explains why orbitals can hold only two electrons.
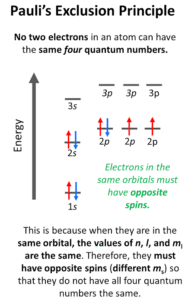
It ensures order and prevents electrons from clashing in the same “space.”
It helps us understand electron configurations, chemical bonding, and the periodic table’s structure.
Summary
- Pauli Exclusion Principle: no two electrons in the same atom can share identical quantum numbers.
- Each orbital can hold two electrons, but their spins must be opposite.
- This principle maintains order and explains the structure of electron shells.
Evaluation
- State the Pauli Exclusion Principle in your own words.
- How many electrons can occupy an orbital according to this principle?
- Why must the spins of two electrons in the same orbital be opposite?
Well done! You’ve just learned one of the strictest “laws” that electrons must obey. Just like order in an exam hall prevents chaos, the Pauli Exclusion Principle ensures order inside the atom. Be proud—you are mastering the invisible rules of nature with Afrilearn by your side.
