Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a delight to learn with you. Picture this: when you’re arranging clothes in your wardrobe, you don’t just throw them in randomly. Shirts go on one side, trousers on another, and shoes in their own section. Similarly, in an atom, electrons don’t just roam about; they are arranged in special regions around the nucleus called atomic orbitals. Each orbital has a unique shape that helps us understand how electrons move and how atoms bond.
Atomic Orbitals And Their Shapes (S, P, D, F)
What are Atomic Orbitals?
An atomic orbital is the region around the nucleus where there is a high probability of finding an electron. Instead of imagining electrons moving in neat circles like Bohr suggested, scientists discovered that electrons actually occupy 3D spaces with different shapes. These shapes are represented as s, p, d, and f orbitals.

s-Orbital
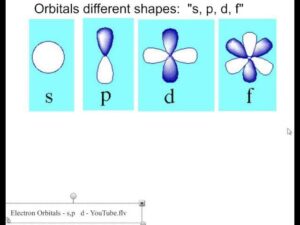
The s-orbital is spherical in shape—just like a football.
Every energy level has one s-orbital.
It can hold a maximum of 2 electrons.
Example: The 1s orbital of hydrogen has its single electron moving in a spherical region around the nucleus.
p-Orbitals
The p-orbitals are dumbbell-shaped, like two balloons tied at the ends.
There are three p-orbitals (px, py, pz), each oriented along the x, y, and z axes.
Together, they can hold up to 6 electrons.
Example: In oxygen (Z = 8), the outer electrons occupy the 2p orbitals, which help oxygen bond strongly with other atoms.
d-Orbitals
The d-orbitals are more complex, often described as clover-shaped.
There are five d-orbitals in each d-subshell.
They can hold a total of 10 electrons.
Example: Transition metals like iron (Fe) have electrons in their 3d orbitals, which give them special properties like magnetism.
f-Orbitals
The f-orbitals are even more complicated in shape, with multi-lobed patterns.
Each f-subshell has seven orbitals.
They can hold up to 14 electrons.
Example: Elements like uranium and other lanthanides and actinides have electrons in their f-orbitals.
Why Are Orbital Shapes Important?
They explain how atoms form bonds. For instance, p-orbitals overlap in bonding to create double and triple bonds.
They help us understand why elements in the same group of the periodic table behave similarly.
They explain properties like magnetism, conductivity, and colour in compounds.
Summary
- Atomic orbitals are regions where electrons are most likely to be found.
- s-orbital: spherical, holds 2 electrons.
- p-orbitals: dumbbell-shaped, three in number, hold 6 electrons.
- d-orbitals: clover-shaped, five in number, hold 10 electrons.
- f-orbitals: complex shapes, seven in number, hold 14 electrons.
Evaluation
- What is the shape of the s-orbital?
- How many electrons can the three p-orbitals hold in total?
- Which type of orbital is responsible for the unique properties of transition metals?
Fantastic work today! You’ve just discovered how electrons arrange themselves in different “rooms” within the atom. Remember, every shape tells us something about the beauty and complexity of matter. With Afrilearn by your side, you’re unlocking the secrets of Chemistry one step at a time.
