Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m so glad to see you again. Let’s begin with something you know well. Imagine a secondary school roll call: the teacher calls students’ names in order of their admission numbers. The list is neat, no confusion, and everyone’s place is clear. If the teacher instead arranged students by height or weight, things would look messy because some tall students may have lower admission numbers. The same thing happened in Chemistry—scientists needed the right order to arrange the elements. That “right order” gave rise to the Modern Periodic Law.
Modern Periodic Law
From Mendeleev to Modern Law
Mendeleev had earlier arranged elements according to atomic mass. His table worked well, but there were problems. For example, argon (atomic mass 40) and potassium (atomic mass 39) seemed out of place because their chemical properties did not fit the order of masses.
In 1913, Henry Moseley, a young British physicist, studied X-ray spectra of elements. He discovered that the identity of an element depends not on its atomic mass but on the number of protons in its nucleus, which we now call the atomic number.
The Modern Periodic Law
The modern periodic law states:
The physical and chemical properties of elements are a periodic function of their atomic numbers.
This means that when elements are arranged in increasing order of atomic number, elements with similar properties appear at regular intervals (periodically).
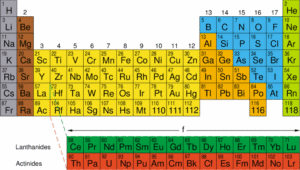
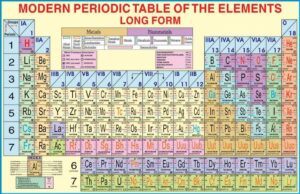
The Modern Periodic Table
The table is arranged in rows called periods and columns called groups.
Elements in the same group have similar outer electron configurations, which explains why they show similar chemical behaviour.
For example, the alkali metals (Group 1: Li, Na, K, Rb, Cs) all react vigorously with water and form +1 ions.
Advantages of the Modern Periodic Table
It corrected the anomalies in Mendeleev’s arrangement (e.g., argon and potassium now fit perfectly).
It provided a clear reason why properties repeat—because of repeating outer electron configurations.
It allowed new elements to be placed in their correct positions without confusion.
Examples in Real Life
Just like students in the same class group often share similarities in age, interests, or subjects, elements in the same group behave alike. For instance, chlorine, bromine, and iodine (all halogens) are used in disinfectants and bleaches, though their strength decreases down the group.
Summary
- Mendeleev arranged elements by atomic mass, but some anomalies existed.
- Moseley discovered atomic number, leading to the modern periodic law.
- Modern periodic law: properties of elements are periodic functions of their atomic numbers.
- This arrangement explains trends and similarities across the table.
Evaluation
- State the modern periodic law.
- Why was Mendeleev’s periodic law replaced?
- Give two advantages of arranging elements by atomic number instead of atomic mass.
Excellent work today! You now understand the foundation of the modern periodic table. Just like order in a school register brings clarity, the modern periodic law brought order to Chemistry. Stay confident—Afrilearn is here to make you love learning more with every lesson.
