Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m glad to have you here today. Let’s begin with something familiar. Imagine you are holding a ₦500 note tightly in your hand. If a friend tries to collect it from you, the effort needed depends on how tightly you’re gripping it. If you hold it loosely, they can take it easily. But if you grip it tightly, they’ll struggle. This is exactly how electrons behave in atoms—the strength with which an atom holds its electrons determines how much energy is required to remove one. This energy is called ionisation energy.
Trends In Ionization Energy
What is Ionisation Energy?
Ionisation energy is the amount of energy required to remove the most loosely bound electron (usually from the outermost shell) from a neutral gaseous atom.
For example:
Na(g) → Na⁺(g) + e⁻ ; ΔH = +496 kJ/mol
Here, 496 kJ/mol is the ionisation energy of sodium.
Factors Affecting Ionisation Energy
Nuclear charge: The more protons in the nucleus, the stronger the pull on electrons, and the higher the ionisation energy.
Atomic radius: Larger atoms have outer electrons farther from the nucleus, so they are removed more easily → lower ionisation energy.
Shielding effect: Inner electrons repel outer ones, reducing nuclear pull. More shielding lowers ionisation energy.
Electron configuration: Atoms with stable configurations (full or half-full subshells) have higher ionisation energies.
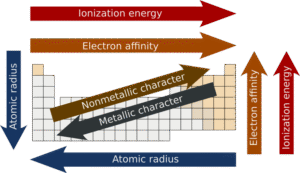
Trends in the Periodic Table
Across a period (left to right): Ionisation energy increases.
Why? Atomic number increases, nuclear charge becomes stronger, and atomic radius decreases. For example, lithium has lower ionisation energy than fluorine.
Down a group (top to bottom): Ionisation energy decreases.
Why? Atoms get bigger, electrons are farther from the nucleus, and shielding increases. For example, potassium has lower ionisation energy than lithium.
Examples in Real Life
Alkali metals (like sodium and potassium) have low ionisation energies, which is why they react quickly and lose electrons easily to form +1 ions.
Noble gases, like neon and argon, have very high ionisation energies because their electron shells are full and very stable.
Summary
- Ionisation energy is the energy needed to remove an electron from a gaseous atom.
- It increases across a period and decreases down a group.
- Factors include nuclear charge, atomic radius, shielding, and electron configuration.
- Low ionisation energy = easy reactivity (like alkali metals).
- High ionisation energy = stability (like noble gases).
Evaluation
- Define ionisation energy in your own words.
- Explain why ionisation energy increases across a period.
- Which has higher ionisation energy: sodium or chlorine? Why?
Excellent! You now understand how atoms “hold on” to their electrons, and how this holding strength changes across the periodic table. Remember, mastering these trends will help you understand chemical reactivity more deeply. Keep going—Afrilearn is here to make Chemistry clear, simple, and exciting for you!
