Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a pleasure to see you here. Let’s start with something relatable. Imagine two friends sharing a plate of jollof rice at a party. One friend keeps pulling the meat closer to their side, while the other just manages with the rice. Even though they are “sharing,” one person clearly has more pulling power. In Chemistry, atoms also “share” electrons when they form bonds, but some atoms pull the shared electrons closer to themselves. This pulling power is called electronegativity.
Electronegativity
What is Electronegativity?
Electronegativity is the tendency of an atom in a chemical bond to attract shared electrons towards itself.
The concept was introduced by Linus Pauling, who also developed a scale (the Pauling scale) to measure electronegativity values. On this scale, fluorine is the most electronegative element with a value of 4.0.
Understanding Electronegativity
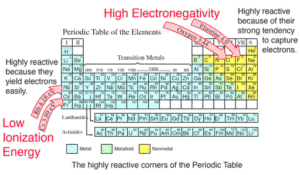
Atoms with high electronegativity strongly attract electrons in a bond (e.g., fluorine, oxygen, chlorine).
Atoms with low electronegativity are weak at attracting shared electrons (e.g., sodium, potassium).
Electronegativity is not a fixed property of an isolated atom—it is meaningful only when the atom is bonded to another.
Factors Affecting Electronegativity
Atomic size: Smaller atoms pull electrons more strongly, so electronegativity is higher.
Nuclear charge: More protons in the nucleus mean stronger attraction for electrons.
Shielding effect: More inner electrons reduce the pull on shared electrons, lowering electronegativity.
Bonding environment: Electronegativity can vary slightly depending on the type of atoms bonded.
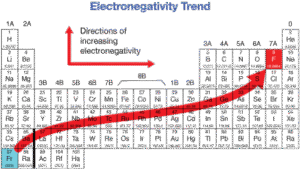
Trends in the Periodic Table
Across a period (left to right): Electronegativity increases. Why? Atomic radius decreases, nuclear charge increases, so atoms attract shared electrons more strongly. Example: carbon < nitrogen < oxygen < fluorine.
Down a group (top to bottom): Electronegativity decreases. Why? Atoms get larger, shielding increases, and the pull on electrons becomes weaker. Example: fluorine > chlorine > bromine > iodine.
Real-Life Examples
In water (H₂O), oxygen is more electronegative than hydrogen, so it pulls electrons closer, making water molecules polar. This is why water dissolves many substances.
Sodium chloride (NaCl) forms because sodium (low electronegativity) loses an electron while chlorine (high electronegativity) strongly attracts it.
Summary
- Electronegativity = the ability of an atom in a bond to attract electrons.
- Pauling scale is the common measure, with fluorine as the highest (4.0).
- It increases across a period, decreases down a group.
- High electronegativity elements are non-metals; low ones are metals.
Evaluation
- Define electronegativity.
- Which element has the highest electronegativity, and why?
- Explain the trend of electronegativity across a period.
Brilliant work today! You’ve mastered electronegativity—the “pulling power” of atoms. Just like in life where some friends pull food closer, in Chemistry, some atoms pull electrons closer. With Afrilearn, you are learning these invisible forces in a clear and exciting way. Keep shining—you’re becoming a true Chemistry star!
