Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a delight to see you here. Let’s begin with something simple and relatable. Imagine the way Nigerian foods change depending on the state. For instance, jollof rice cooked in Lagos might be slightly different from that in Enugu or Kano—same idea, different style. In Chemistry, oxides and hydrides of elements also show a “pattern of change” as you move across periods or down groups in the periodic table. This repeating pattern is what we call periodicity.
Periodicity In Oxides And Hydrides
What are Oxides and Hydrides?
Oxides are compounds formed when an element combines with oxygen. Example: Na₂O (sodium oxide), CO₂ (carbon dioxide).
Hydrides are compounds formed when an element combines with hydrogen. Example: NaH (sodium hydride), NH₃ (ammonia).
Periodicity in Oxides
The properties of oxides change systematically across a period and down a group.
Across a Period (left to right):
Oxides of metals on the left (e.g., Na₂O, MgO) are basic. They react with water to form alkaline solutions (Na₂O + H₂O → 2NaOH).
Oxides of non-metals on the right (e.g., CO₂, SO₂) are acidic. They react with water to form acids (SO₂ + H₂O → H₂SO₃).
Oxides of elements near the middle (like Al₂O₃, SiO₂) are amphoteric or neutral.
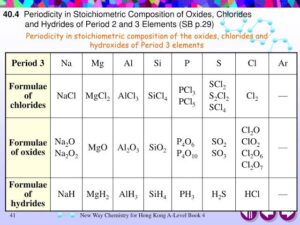
Example: Period 3 oxides show this progression:
Na₂O (basic) → MgO (basic) → Al₂O₃ (amphoteric) → SiO₂ (acidic) → P₄O₁₀ (acidic) → SO₃ (acidic) → Cl₂O₇ (acidic).
Down a Group:
Oxides become more basic as metallic character increases.
Example: In Group 2, MgO is basic, CaO is strongly basic, and BaO is even more basic.
Periodicity in Hydrides
Across a Period:
Hydrides of metals on the left (NaH, MgH₂) are ionic and act as strong bases.
Hydrides of non-metals (CH₄, NH₃, H₂O, HF) are covalent. Their properties vary depending on electronegativity and hydrogen bonding.
Example: From methane (CH₄) to hydrogen fluoride (HF), boiling points rise due to increasing hydrogen bonding strength.
Down a Group:
In Group 14 (CH₄, SiH₄, GeH₄, SnH₄), stability of hydrides decreases as you go down.
In Group 16 (H₂O, H₂S, H₂Se, H₂Te), boiling points increase down the group due to increasing molecular mass, except water which is unusually high due to strong hydrogen bonding.
Everyday Connections
The strong base NaOH formed from sodium oxide is used in making soap and detergents in Nigeria.
Acidic oxides like CO₂ play a role in carbonated drinks and also in climate change discussions.
Hydrides like NH₃ (ammonia) are essential for fertilisers used in farming across Africa.
Summary
- Oxides can be basic, acidic, amphoteric, or neutral depending on the element.
- Across a period: oxides change from basic (metals) → amphoteric → acidic (non-metals).
- Down a group: oxides become more basic.
- Across a period: hydrides change from ionic (metals) to covalent (non-metals).
- Down a group: hydrides’ stability decreases, while boiling points may increase.
Evaluation
- State the trend in the nature of oxides across a period.
- Why is H₂O’s boiling point unusually high compared to H₂S?
- Write the balanced chemical equation for the reaction between sodium oxide and water.
Excellent work today! You’ve just learnt how oxides and hydrides follow predictable patterns, just like recipes that vary across Nigeria but still keep their essence. With Afrilearn, Chemistry becomes as relatable as the food you love and the world you live in. Keep shining—you’re mastering patterns like a true scientist!
