Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s great to have you here again. Let’s begin with something you can easily picture. Imagine blocks of sugar or cubes of salt—hard, solid, and easy to hold. Both are examples of crystalline materials. Now, one of them, table salt (NaCl), is an ionic compound. Its unique properties come from the strong attraction between oppositely charged ions. Today, we’ll look at the special features that make ionic compounds different from others.
Properties Of Ionic Compounds
What are Ionic Compounds?
Ionic compounds are substances formed when metals transfer electrons to non-metals, creating positive and negative ions held together by strong electrostatic forces. Examples include sodium chloride (NaCl), magnesium oxide (MgO), and calcium fluoride (CaF₂).
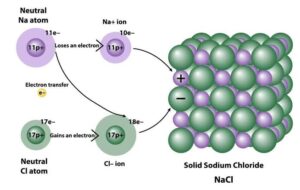
Properties of Ionic Compounds
High Melting and Boiling Points
The electrostatic forces between cations and anions are very strong, requiring a lot of heat energy to break.
Example: NaCl has a melting point of about 801 °C.
Solid and Crystalline at Room Temperature
Ionic compounds form regular repeating structures (crystals).
This makes them hard and brittle.
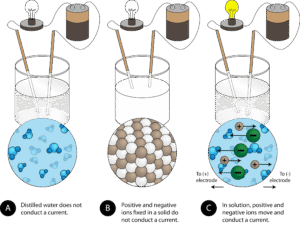
Electrical Conductivity
In the solid state, ionic compounds do not conduct electricity because ions are fixed in place.
When melted or dissolved in water, the ions are free to move and carry current.
Example: Molten NaCl conducts electricity and is used in electrolysis.
Solubility in Water
Many ionic compounds dissolve easily in water because water molecules attract and pull the ions apart.
Example: NaCl dissolves in water, making the salty water we taste.
Brittleness
Ionic compounds can shatter when struck because similar charges get pushed close together, causing repulsion.
Everyday Connections
Table salt (NaCl) adds flavour to your food—its solubility in water is what makes it useful.
Electrolysis of molten NaCl produces sodium (used in streetlights) and chlorine (used in water purification).
MgO, an ionic compound, is used to make heat-resistant bricks for furnaces.
Summary
- Ionic compounds are made up of positive and negative ions held together by strong electrostatic forces.
- They have high melting and boiling points, are usually hard and crystalline, and are soluble in water.
- They conduct electricity in molten or aqueous form but not as solids.
- They are brittle and can break when struck.
Evaluation
- Why do ionic compounds have high melting points?
- Explain why NaCl does not conduct electricity in solid state but does when dissolved in water.
- Give two uses of ionic compounds in everyday life.
Fantastic work today! You’ve just seen how ionic compounds behave in the lab and in your daily life. With Afrilearn, Chemistry is no longer abstract—it’s connected to the salt in your food, the electricity around you, and the materials in your environment. Keep going; you’re doing brilliantly!
