Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s a joy to have you here again. Let’s begin with a picture you know well. Think about Nigerian jollof rice. Sometimes you taste it and notice more tomato flavour, other times more pepper—it still remains jollof rice, but the taste can vary slightly depending on the mixture. In Chemistry, certain molecules also exist in different “forms” that are not fixed but blended together. This is called resonance. Today, we’ll also talk about bond polarity and bond energy—all key ideas that explain how molecules behave.
Resonance, Bond Polarity, Bond Energy
Resonance
Some molecules cannot be represented by just one Lewis structure. Instead, they have two or more possible structures that combine to form a “resonance hybrid.”
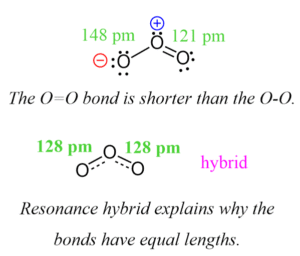
Example: Ozone (O₃)
You can draw O₃ with a double bond on the left and a single bond on the right, or vice versa.
The real structure is a blend, with bonds of equal length.
Example: Benzene (C₆H₆)
It can be drawn with alternating double and single bonds, but in reality, all bonds are equal.
Resonance makes molecules more stable, just like a well-balanced recipe keeps food tasting better.
Bond Polarity
A covalent bond is not always a perfect “sharing” of electrons. Sometimes, one atom attracts the shared electrons more strongly, creating a polar bond.
If both atoms share equally → Non-polar bond (e.g., H₂, O₂).
If one atom pulls harder → Polar bond (e.g., H–Cl, where chlorine attracts electrons more).
Polarity arises from electronegativity difference between the atoms.
Larger difference = more polar bond.
Example: In water (H₂O), oxygen pulls electrons more strongly, making water polar. This explains why water dissolves salts and sugars easily.
Bond Energy
Bond energy is the amount of energy required to break one mole of a particular bond in a gaseous state.
Stronger bonds → higher bond energy.
Multiple bonds (double, triple) have greater bond energy than single bonds.
Example: C≡C triple bond > C=C double bond > C–C single bond.
Bond energy is important because it helps us understand chemical reactions. Reactions occur when old bonds are broken and new ones formed.
Everyday Connections
The stability of medicines and plastics often comes from resonance.
The polar nature of water explains why it’s called the “universal solvent.”
Fuels like petrol release energy because of the bond energies in hydrocarbons.
Summary
- Resonance: Molecules can be represented by two or more Lewis structures; the real structure is a hybrid.
- Bond polarity: Unequal sharing of electrons due to electronegativity difference.
- Bond energy: Energy required to break a bond; stronger bonds have higher bond energy.
Evaluation
- Define resonance and give one example.
- Explain why HCl is a polar molecule.
- Arrange the following bonds in order of increasing bond energy: C≡C, C–C, C=C.
Fantastic work today! You’ve learnt how molecules “blend,” how they can have “unequal sharing” of electrons, and how much energy it takes to break bonds. With Afrilearn, you’re building real confidence in Chemistry—step by step, you’re mastering it. Keep shining; the next lesson will be even more exciting!
