Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m glad you’re here again. Let’s begin with something familiar. Imagine you and your friends trying to sit around a small round table at a buka. Everyone tries to sit as far apart as possible so they’ll have enough space and not bump into each other’s elbows. Atoms behave the same way in molecules—electron pairs around a central atom push each other apart, arranging themselves to minimise repulsion. This simple idea is the heart of the VSEPR theory.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
What is VSEPR Theory?
VSEPR stands for Valence Shell Electron Pair Repulsion theory. It states that:
Electron pairs around a central atom repel one another.
They arrange themselves as far apart as possible to minimise repulsion.
This determines the shape (geometry) of the molecule.
Types of Electron Pairs
Bonding pairs: Shared between two atoms (form covalent bonds).
Lone pairs: Not shared, belong only to one atom.
Lone pairs repel more strongly than bonding pairs, so they influence molecular shape significantly.
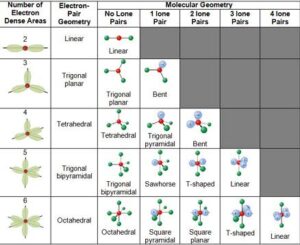
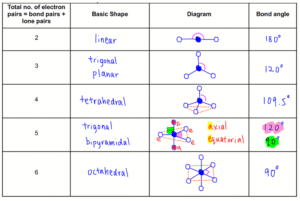
Common Molecular Shapes (with examples)
Linear (180°):
2 bonding pairs, no lone pairs.
Example: CO₂ (O=C=O).
Trigonal Planar (120°):
3 bonding pairs, no lone pairs.
Example: BF₃.
Tetrahedral (109.5°):
4 bonding pairs, no lone pairs.
Example: CH₄ (methane).
Trigonal Pyramidal (~107°):
3 bonding pairs, 1 lone pair.
Example: NH₃ (ammonia).
Bent/V-shaped (~104.5°):
2 bonding pairs, 2 lone pairs.
Example: H₂O (water).
Trigonal Bipyramidal (90° & 120°):
5 bonding pairs.
Example: PCl₅.
Octahedral (90°):
6 bonding pairs.
Example: SF₆.
Everyday Connections
The shape of water (bent) explains why it is a polar molecule and a good solvent.
The structure of carbon compounds like methane (CH₄) is the foundation of fuels, plastics, and even DNA structures.
Medicines depend on molecular shape to fit correctly into body receptors.
Summary
- VSEPR theory explains molecular shapes based on repulsion between electron pairs.
- Lone pairs push harder than bonding pairs, changing bond angles.
- Common shapes include linear, trigonal planar, tetrahedral, pyramidal, bent, trigonal bipyramidal, and octahedral.
Evaluation
- State the main idea of VSEPR theory.
- Predict the shape of ammonia (NH₃).
- Why does water (H₂O) have a bent shape instead of linear?
- Draw and name the shape of methane (CH₄).
You’ve done wonderfully today! Now you can “see” molecules not just as formulas, but as real 3D structures with shapes and angles. With Afrilearn, you’re gaining the confidence to handle Chemistry like a pro. Keep up the amazing effort—the next lesson will add even more colour to your learning journey!
