Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m happy to have you here once again. Let’s begin with something you can picture. Imagine you have different Nigerian musical instruments—like the talking drum, flute, and shekere. On their own, each makes a unique sound. But when you blend them together in harmony, you get a richer, more balanced sound that powers a party. In Chemistry, something similar happens: atomic orbitals (s, p, and sometimes d) mix to form new “hybrid orbitals” that give atoms the ability to bond in specific shapes. This is called hybridisation.
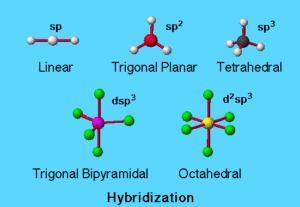
Hybrid Orbitals (Sp, Sp², Sp³, Dsp³, D²sp³)
What is Hybridisation?
Hybridisation is the process of mixing atomic orbitals (s, p, and sometimes d) on the same atom to form new orbitals with equal energy (degenerate orbitals). These new orbitals determine the shape of molecules.
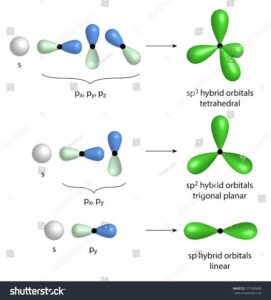
Types of Hybrid Orbitals
1. sp Hybridisation
Combination: one s orbital + one p orbital.
Number of hybrid orbitals: 2.
Shape: Linear, bond angle = 180°.
Example: CO₂ (O=C=O).
Picture it like two people standing at opposite ends of a long bench, as far apart as possible.
2. sp² Hybridisation
Combination: one s orbital + two p orbitals.
Number of hybrid orbitals: 3.
Shape: Trigonal planar, bond angle = 120°.
Example: BF₃, ethene (C₂H₄).
Think of three friends standing around a suya stand at equal angles.
3. sp³ Hybridisation
Combination: one s orbital + three p orbitals.
Number of hybrid orbitals: 4.
Shape: Tetrahedral, bond angle = 109.5°.
Example: CH₄ (methane).
Just like four legs of a chair are evenly spaced to keep it stable.
4. dsp³ Hybridisation
Combination: one d orbital + one s orbital + three p orbitals.
Number of hybrid orbitals: 5.
Shape: Trigonal bipyramidal (two bonds at 90°, three at 120°).
Example: PCl₅.
Imagine three people forming a triangle at ground level while two others stand directly above and below them.
5. d²sp³ Hybridisation
Combination: two d orbitals + one s orbital + three p orbitals.
Number of hybrid orbitals: 6.
Shape: Octahedral, bond angle = 90°.
Example: SF₆.
Think of six friends forming a perfect cube arrangement, one on each corner.
Everyday Connections
The stability of organic molecules like methane depends on hybridisation.
The flatness of benzene (sp² hybridisation) is why it’s used in making plastics and dyes.
The unique stability of SF₆ (d²sp³) is why it’s used in Nigerian power stations as an insulating gas.
Summary
- Hybridisation = mixing of atomic orbitals to form new orbitals of equal energy.
- sp (linear, 180°), sp² (trigonal planar, 120°), sp³ (tetrahedral, 109.5°), dsp³ (trigonal bipyramidal), d²sp³ (octahedral).
- Molecular shape and bonding are deeply linked to hybridisation.
Evaluation
- Define hybridisation in your own words.
- State the hybridisation and shape of CH₄.
- Which molecule shows sp² hybridisation: CO₂, BF₃, or CH₄?
- How many hybrid orbitals are formed in dsp³ hybridisation?
Excellent effort today! You’ve learnt how orbitals mix just like instruments in a band, giving molecules their shape and stability. With Afrilearn, you’re not only understanding Chemistry—you’re enjoying the rhythm of learning. Stay excited, because your next lesson will take you even further!
