Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a joy to have you here. Let’s begin with a simple thought: Imagine you’re listening to your favourite Afrobeat song through an old, crackling radio. The music is there, but it doesn’t sound as rich as when played through modern speakers. In the same way, Valence Bond Theory (VBT) is useful—it explains chemical bonding—but it has its own “crackles” or weaknesses. Scientists later improved on it with more advanced theories. Today, let’s look at the limitations of VBT.
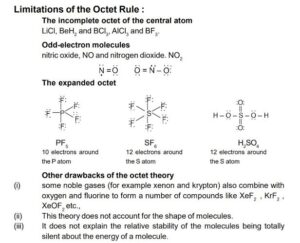
Limitations
Limitations of Valence Bond Theory
Failure to Explain Magnetic Properties
VBT cannot explain why some molecules are magnetic and others are not.
Example: Oxygen (O₂) is known to be paramagnetic (attracted to a magnet), but according to VBT, it should be diamagnetic (not attracted). This mismatch shows VBT is incomplete.
Inability to Explain Spectra of Molecules
VBT does not explain the absorption or emission spectra of molecules.
Scientists need a theory that connects bonding with light interaction (later explained by Molecular Orbital Theory).
Does Not Account for Delocalisation of Electrons
VBT assumes electrons are always localised between two atoms in a bond.
But in reality, some molecules (like benzene, C₆H₆) have delocalised electrons spread across several atoms.
This is why benzene is unusually stable, something VBT struggles to explain.
No Clear Explanation for Bond Energies
VBT talks about bonds forming through orbital overlap but does not precisely calculate bond energies or bond lengths.
For example, it cannot easily explain why a C=O double bond is shorter and stronger than a C–O single bond.
Shapes of Some Molecules
VBT explains some molecular shapes through hybridisation (e.g., CH₄ as tetrahedral), but it does not always succeed for molecules involving d-orbitals or complex ions.
For example, it cannot properly explain the bonding in transition metal complexes like [Fe(CN)₆]³⁻.
Everyday Connection
Think of VBT like a black-and-white TV. It helped people watch films, but it lacked colour, clarity, and detail. Just like television technology improved to HD and 4K, Chemistry also needed a better theory—this led to the Molecular Orbital Theory (MOT), which explains bonding, magnetism, spectra, and delocalisation more clearly.
Summary
- VBT is helpful but incomplete.
- It fails to explain magnetism, spectra, delocalisation of electrons, bond energies, and some molecular shapes.
- These weaknesses gave rise to more advanced theories, especially Molecular Orbital Theory.
Evaluation
- Mention two properties of molecules that VBT fails to explain.
- Why is oxygen (O₂) a limitation for VBT?
- Explain briefly why benzene cannot be explained fully by VBT.
- Which advanced theory was developed to correct the shortcomings of VBT?
Fantastic work! You now know that even powerful theories have their limits, and science keeps improving. With Afrilearn, you’re building not just knowledge, but also the wisdom to ask deeper questions. Keep shining—you’re doing excellently!
