Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m so happy you’re here again. Imagine two streams in a Nigerian village flowing side by side. When they meet, the waters mix and form one bigger river. You can no longer say, “This part is stream A” or “That part is stream B”—the waters are now shared. This is exactly what happens when atomic orbitals combine to form molecular orbitals. Today, we’ll learn how molecular orbitals are formed.
Formation Of Molecular Orbitals
What are Molecular Orbitals?
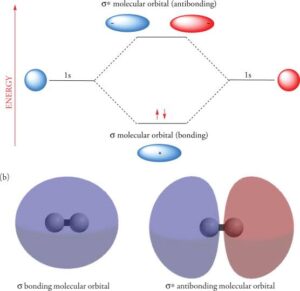
A molecular orbital is formed when atomic orbitals from two atoms overlap and combine. Unlike atomic orbitals, which belong to a single atom, molecular orbitals belong to the entire molecule. Electrons in molecular orbitals are shared by all the nuclei in the molecule.
Principles of Molecular Orbital Formation
Combination of Atomic Orbitals
When two atomic orbitals of similar energy and symmetry overlap, they form two molecular orbitals:
Bonding Molecular Orbital (lower energy, stable).
Antibonding Molecular Orbital (higher energy, unstable).
Constructive Interference (Bonding MO)
If the overlapping wave functions reinforce each other, a bonding orbital forms.
This increases electron density between the two nuclei, pulling them together.
Everyday picture: Two friends working together to push a car—they succeed faster.
Destructive Interference (Antibonding MO)
If the overlapping wave functions cancel each other out, an antibonding orbital forms.
This reduces electron density between the nuclei, making the bond weaker.
Everyday picture: Two people pushing a car in opposite directions—it doesn’t move.
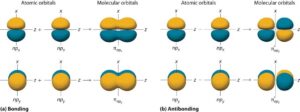
Types of Molecular Orbitals
Sigma (σ) molecular orbitals: Formed by head-on overlap along the axis joining two nuclei. Example: overlap of 1s–1s in H₂.
Pi (π) molecular orbitals: Formed by sideways overlap of p orbitals. Example: p–p overlap in O₂.
Important Rules of Molecular Orbital Theory
The number of molecular orbitals formed equals the number of atomic orbitals combined.
Bonding orbitals are always lower in energy, antibonding orbitals are higher.
Electrons fill molecular orbitals according to Aufbau principle, Hund’s rule, and Pauli exclusion principle.
Everyday Connections
The concept of molecular orbitals explains why oxygen (O₂) is magnetic, something VBT failed to explain.
Industries in Nigeria making fertilisers and fuels rely on MOT to understand molecules like N₂ and CO₂ at a deeper level.
Just as teamwork brings greater results, constructive orbital overlap brings stronger bonds.
Summary
- Molecular orbitals form when atomic orbitals overlap.
- Two types of molecular orbitals: bonding (stable) and antibonding (unstable).
- Types of overlaps: sigma (head-on) and pi (sideways).
- MOT explains many properties VBT could not, like magnetism and bond stability.
Evaluation
- What is a molecular orbital?
- Differentiate between bonding and antibonding molecular orbitals.
- State one difference between sigma and pi molecular orbitals.
- Why is oxygen (O₂) paramagnetic according to MOT?
Excellent learning today! You’ve just unlocked how orbitals “merge” to form molecular orbitals, giving Chemistry a deeper rhythm. With Afrilearn, every lesson brings clarity and confidence. Keep your energy up—we’re moving into exciting applications of this theory next!
