Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m so glad to see you today. Picture this: two friends in Abuja decide to carry a heavy bag of rice together. By joining their strength, the load feels lighter and easier to carry. But if those same two friends pull the bag in opposite directions, they waste energy and the bag doesn’t move forward. This everyday scene captures the difference between bonding orbitals and antibonding orbitals in Chemistry.
Bonding And Antibonding Orbitals
Bonding Molecular Orbitals
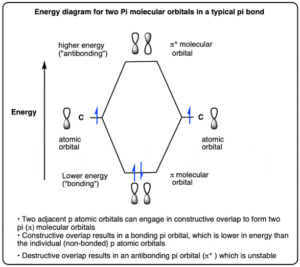
Bonding orbitals are formed when atomic orbitals combine in such a way that the overlap increases electron density between the two nuclei.
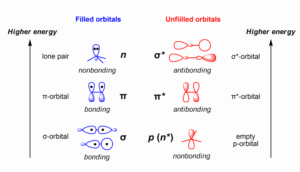
This creates a region of strong attraction that holds the atoms together.
Bonding orbitals are lower in energy than the original atomic orbitals, making the molecule more stable.
Key Features of Bonding Orbitals:
Electrons are concentrated between the nuclei.
Stability of the molecule increases.
Energy is lower compared to the original orbitals.
Everyday link: Like two friends working together in harmony—things get easier and more productive.
Antibonding Molecular Orbitals
Antibonding orbitals form when atomic orbitals combine in such a way that their overlap decreases electron density between the nuclei.
This creates repulsion rather than attraction.
Antibonding orbitals are higher in energy than the original atomic orbitals, making the molecule less stable.
Key Features of Antibonding Orbitals:
Electrons are concentrated away from the nuclei (a nodal plane appears between them).
Stability of the molecule decreases.
Energy is higher compared to the original orbitals.
Everyday link: Like two people dragging a bag in opposite directions—it wastes energy and creates tension.
Symbols Used
Bonding orbitals are labelled as σ (sigma) or π (pi).
Antibonding orbitals are written as σ* or π* (with a star indicating antibonding).
Effect on Molecules
If bonding orbitals are filled with more electrons than antibonding orbitals, the molecule is stable.
If antibonding orbitals have equal or more electrons than bonding orbitals, the molecule becomes unstable.
Everyday Connections
The reason H₂ exists as a stable molecule is because its electrons occupy bonding orbitals.
On the other hand, He₂ does not exist under normal conditions because the electrons occupy both bonding and antibonding orbitals, cancelling each other out.
This idea is important in industries like petrochemicals, where understanding stable and unstable molecules affects reactions.
Summary
- Bonding orbitals form from constructive overlap → stable, lower energy.
- Antibonding orbitals form from destructive overlap → unstable, higher energy.
- Stability of a molecule depends on the balance between electrons in bonding and antibonding orbitals.
Evaluation
- What is the difference between bonding and antibonding orbitals?
- Why are bonding orbitals more stable?
- Why doesn’t He₂ exist as a stable molecule?
- Write the symbols for bonding and antibonding sigma orbitals.
You’ve done brilliantly today! You now understand why some molecules are strong and stable while others fall apart. Keep shining—Afrilearn is here to make Chemistry as easy and exciting as a good story. Your next lesson will take this even further!
