Back to: Inorganic Chemistry 100 Level
Welcome to class!
It feels good to have you here again. Picture this: you’re at a party in Lagos, and even though you don’t know everyone, you still feel the subtle pull of the crowd—someone brushes past you, another person stands too close, and suddenly you’re moving slightly without even planning to. This gentle, temporary attraction is similar to what happens in molecules through van der Waals forces.
Van Der Waals Forces
What are van der Waals Forces?
Van der Waals forces are weak intermolecular forces of attraction between molecules. They are the weakest of all molecular forces but are very important, especially in non-polar molecules where no permanent dipole exists.
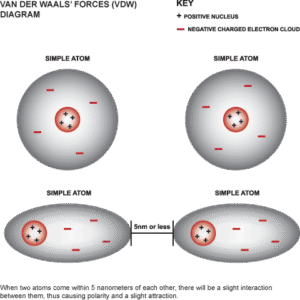
These forces arise from temporary changes in electron distribution around atoms or molecules. Imagine the electrons in an atom not being evenly spread out—sometimes more are on one side, creating a temporary dipole that attracts another atom nearby.
Types of van der Waals Forces
London Dispersion Forces (Instantaneous Dipole-Induced Dipole):
Present in all molecules, whether polar or non-polar.
They arise due to temporary fluctuations in electron density.
Example: In noble gases like argon or non-polar molecules like oxygen (O₂), London forces are the only attractions holding them together.
Dipole-Induced Dipole Forces:
Occur when a polar molecule with a permanent dipole induces a dipole in a neighbouring non-polar molecule.
Example: Oxygen gas (non-polar) can be slightly attracted to water molecules (polar) due to this effect.
Importance of van der Waals Forces
Explains states of matter: Noble gases like helium and argon are gases at room temperature because they only have weak van der Waals forces. Larger noble gases like xenon can become liquids more easily because their van der Waals forces are stronger.
Boiling and melting points: Non-polar molecules like iodine (I₂) are solid at room temperature because larger molecules have stronger dispersion forces, while smaller ones like chlorine (Cl₂) are gases.
Everyday life:
The ability of geckos to climb walls depends partly on van der Waals forces between their feet and the wall.
In cooking gas (butane, propane), these weak forces hold molecules together until they are released for burning.
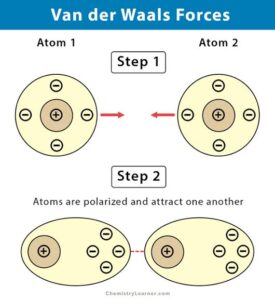
Strength of van der Waals Forces
Weakest compared to dipole–dipole interactions and hydrogen bonding.
Increase with:
Larger number of electrons (bigger atoms/molecules).
Greater molecular surface area for contact.
Summary
- Van der Waals forces are weak intermolecular forces present in all molecules.
- They include London dispersion and dipole-induced dipole interactions.
- They play a key role in determining physical states, solubility, and boiling/melting points.
Evaluation
- What are van der Waals forces in simple terms?
- Explain the difference between London dispersion forces and dipole-induced dipole forces.
- Why does iodine exist as a solid while chlorine exists as a gas at room temperature?
- Give one real-life example of van der Waals forces at work.
Excellent! You’ve mastered another important concept in Chemistry. Remember, even the smallest forces matter in the grand design of nature. With Afrilearn, every lesson is a step closer to your best self.
