Back to: Inorganic Chemistry 100 Level
Welcome to class!
I’m happy to see you here today. Let’s start with something familiar. Think about cooking beans on a Saturday morning in Nigeria. Beans usually take a long time to soften and “melt” when boiled compared to something like indomie noodles, which cook almost instantly. In Chemistry, substances also take longer or shorter times to melt or boil, depending on the types of forces holding their particles together. Similarly, some things dissolve easily in water, like sugar, while others, like palm oil, stubbornly refuse. Today, we’ll learn how intermolecular forces explain these everyday observations through their relation to boiling/melting points and solubility.
Relation To Boiling/melting Points And Solubility
Boiling and Melting Points
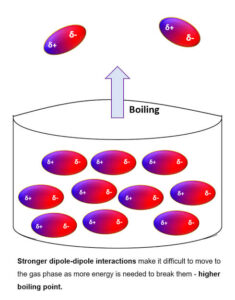
The boiling point of a substance is the temperature at which it changes from liquid to gas.
The melting point is the temperature at which a solid changes into liquid.
Both depend on how strongly the particles are held together by intermolecular forces.
Key Relationships
Stronger intermolecular forces = higher boiling and melting points.
Example: Water (H₂O) has strong hydrogen bonds, so it boils at 100 °C. Compare this to oxygen gas (O₂), held only by weak van der Waals forces, which boils at –183 °C.
Weaker intermolecular forces = lower boiling and melting points.
Example: Noble gases like helium are gases at room temperature because their forces are very weak.
Order of Strength of Forces (and their effect on boiling/melting points):
van der Waals < dipole–dipole < hydrogen bonding < ionic/covalent bonding
This explains why common salt (NaCl), held by ionic bonds, has a very high melting point compared to sugar, which is held by hydrogen bonds.
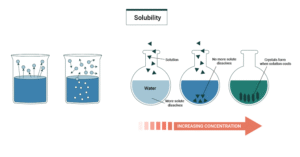
Solubility
Solubility refers to how well a substance dissolves in a solvent (like water). The golden rule is: “like dissolves like.”
Polar substances dissolve best in polar solvents.
Example: Sugar dissolves easily in water because both have hydrogen bonding.
Non-polar substances dissolve best in non-polar solvents.
Example: Palm oil mixes with kerosene but not with water.
If the intermolecular forces between solute and solvent are compatible, solubility is high; if not, solubility is low.
Everyday Nigerian Examples
Why does garri swell in water but not in petrol? Because garri (starch) is polar and matches water’s polarity.
Why is groundnut oil used to fry akara but doesn’t mix with water? Because oil is non-polar, while water is polar.
Why do soft drinks release bubbles when opened? Carbon dioxide dissolves in water due to dipole-induced dipole interactions, but escapes when pressure is released.
Summary
- Stronger intermolecular forces mean higher boiling and melting points.
- Weaker forces mean lower boiling and melting points.
- “Like dissolves like”: polar dissolves in polar, non-polar dissolves in non-polar.
- Everyday cooking, drinks, and oils all reflect these principles.
Evaluation
- Why does water boil at a higher temperature than oxygen gas?
- Compare the boiling points of NaCl and H₂O. Explain why one is higher.
- State the rule for solubility and give one Nigerian food example.
- Why does palm oil not dissolve in water?
Fantastic work today! Remember, Chemistry is not far away in a lab—it’s in your food, your drinks, and even in how substances mix and separate around you. Keep going, you are becoming a true scientist with Afrilearn.
