Back to: Inorganic Chemistry 100 Level
Welcome to class!
It’s always a joy to learn with you. Imagine walking into Balogun Market in Lagos. Every stall has its own arrangement—clothes in one corner, shoes in another, food items in another. This neat arrangement helps customers find what they need easily. The periodic table is like that market—an orderly arrangement of elements based on their atomic structure and properties. To understand why the table looks the way it does, we must first recall what atoms are and then link this to the patterns, or periodic trends, we see across the table.
Review Of Atomic Structure, Periodic Trends
Atomic Structure: A Quick Review
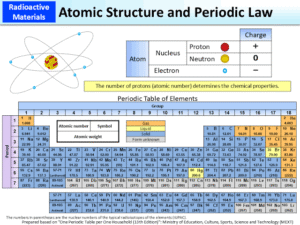
An atom is the basic building block of matter. Its main parts are:
Nucleus: Contains protons (positively charged) and neutrons (neutral). Almost all the mass of the atom is concentrated here.
Electrons: Negatively charged particles that move around the nucleus in regions called orbitals.
Atomic number (Z): Number of protons in the nucleus. This defines the identity of the element.
Mass number (A): Sum of protons and neutrons.
Electrons are arranged in shells and subshells, following rules like the Aufbau principle, Hund’s rule, and Pauli exclusion principle. This arrangement is what gives each element its unique properties.
Periodic Trends
Because of the way electrons are arranged, elements show patterns (trends) in their properties as you move across a period (left to right) or down a group (top to bottom).
Atomic Radius:
Across a period: decreases (because more protons pull the electrons closer).
Down a group: increases (because more shells are added).
Example: Sodium (Na) is bigger than chlorine (Cl), even though Cl is to the right in the same period.
Ionisation Energy (energy needed to remove an electron):
Across a period: increases (harder to remove electrons due to stronger nuclear pull).
Down a group: decreases (electrons are farther from the nucleus and easier to remove).
Electronegativity (ability of an atom to attract electrons):
Across a period: increases.
Down a group: decreases.
Example: Oxygen is more electronegative than sodium.
Electron Affinity (energy released when an electron is added):
Becomes more negative across a period.
Becomes less negative down a group.
Everyday Connection
Salt (NaCl) forms because sodium, with low electronegativity, easily gives up an electron, while chlorine, with high electronegativity, loves to gain one.
Oxygen in our air supports breathing and burning because of its high electronegativity, which makes it eager to form bonds.
The reason cooking gas (butane, C₄H₁₀) and metals behave so differently is linked to where they fall on the periodic table and their atomic structure.
Summary
- Atoms are made of protons, neutrons, and electrons.
- The arrangement of electrons determines chemical properties.
- Periodic trends include atomic radius, ionisation energy, electronegativity, and electron affinity.
- These trends explain why elements behave differently across the table.
Evaluation
- What is the difference between atomic number and mass number?
- Why does atomic radius decrease across a period but increase down a group?
- Which element has a higher electronegativity: sodium or chlorine? Why?
- How do periodic trends explain the formation of NaCl?
Fantastic work today! You’ve just revised the foundation that connects atomic structure to periodic trends. With this knowledge, the periodic table will no longer look like a confusing chart—it’s now your roadmap to understanding Chemistry. Keep going, Afrilearn is proud of you!
