Back to: Organic Chemistry 400 Level
Welcome to class!
Hello brilliant learner, I’m really happy to have you here again. I hope your mind is alert and ready, because today’s lesson — Advanced Reaction Mechanisms — will help you deepen your understanding of how organic reactions truly occur at the molecular level. At this stage, it’s not just about knowing what the reactants and products are, but understanding why a particular pathway is followed and how each step proceeds. This will help you confidently predict reaction outcomes and design new synthetic routes in the future.
Advanced Reaction Mechanisms
Have you ever wondered why two different reactants sometimes give the same product through different routes, or why some reactions take place rapidly while others are very slow? These differences are explained by advanced reaction mechanisms.
Reaction Energy Profile and Transition States
A reaction energy profile is a graph that shows how the energy of a system changes during a reaction.
Reactants start at a certain energy level.
Transition states are high-energy points that represent unstable arrangements of atoms.
Activation energy (Ea) is the energy required to reach the transition state.
Intermediates lie in energy minima between two transition states.
Understanding this profile enables chemists to explain why some reactions are faster or slower, and how catalysts can lower the activation energy to speed up the reaction.
Reaction Pathways: Stepwise vs. Concerted Mechanisms
Stepwise mechanisms involve two or more distinct steps, each with its own intermediate (e.g., electrophilic addition to alkenes).
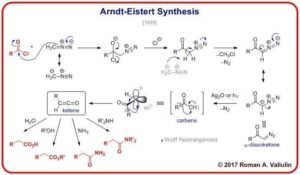
Concerted mechanisms occur in a single step without intermediates (e.g. pericyclic reactions such as the Diels–Alder reaction).
You can compare stepwise reactions to cooking a complex meal where different ingredients are added in stages, while concerted reactions are like preparing instant noodles — everything happens in one smooth action.
Reaction Rate and Rate-Determining Step
The rate-determining step (RDS) is the slowest step in a multi-step mechanism and controls the overall rate of reaction. It is similar to traffic on a badly damaged section of a road – no matter how smooth the rest of the road is, that one section slows down the entire journey.
Reactive Intermediates and Their Stability
Several intermediates can be formed in advanced mechanisms:
Carbocations (stabilised by alkyl groups and resonance)
Carbanions (stabilised by electron-withdrawing groups)
Free radicals (involved in halogenation and polymerisation)
Carbenes (used in cyclopropanation reactions)
The stability of these intermediates determines which pathway is favoured. For example, a tertiary carbocation is more stable than a secondary one, so mechanisms that lead to tertiary carbocation often proceed more readily.
Kinetic vs. Thermodynamic Control
Kinetic control favours the product that forms fastest (usually at lower temperatures).
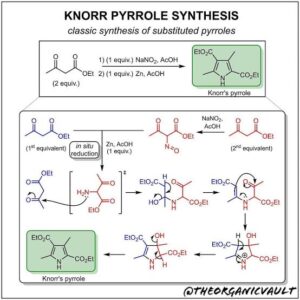
Thermodynamic control favours the most stable product (usually at higher temperatures).
Imagine frying plantain quickly on high heat (kinetic) or slow-roasting it to bring out a richer flavour (thermodynamic). Both processes give fried plantain, but one is quicker while the other is more stable and flavourful.
Summary
- Advanced reaction mechanisms analyse how and why reactions proceed via transition states and intermediates.
- Energy profiles show the energy changes along a reaction pathway and help identify activation energy and transition states.
- Stepwise mechanisms involve multiple stages with intermediates, while concerted mechanisms occur in one step.
- The rate-determining step is the slowest step and controls the overall reaction rate.
- Kinetic and thermodynamic control determine whether the reaction favours faster or more stable products.
Evaluation
- What is a transition state and how is it related to activation energy?
- Differentiate between stepwise and concerted mechanisms.
- Explain what is meant by the rate-determining step.
- Give one example each of kinetic control and thermodynamic control in organic reactions.
Excellent work today! Your understanding of organic chemistry is growing stronger every day. Keep studying confidently — Afrilearn is proud to support you on this exciting journey!
