Back to: Organic Chemistry 200 Level
Welcome to class!
Hello, champ! I’m so glad you’re here today. We’re about to learn something truly exciting — Alcohols, Phenols, and Ethers. You’ve definitely come across some of these in your everyday life. That bottle of methylated spirit at home? That’s an alcohol. The antiseptic Dettol? That contains phenol. Even perfumes and some medicines contain ethers. So yes — these are not just chemicals in your textbook, they are part of your world. Let’s walk through this together, one step at a time.
Alcohols, Phenols & Ethers
What Are Alcohols, Phenols, and Ethers?
These are all organic compounds that contain oxygen, but they behave quite differently because of how that oxygen is bonded.
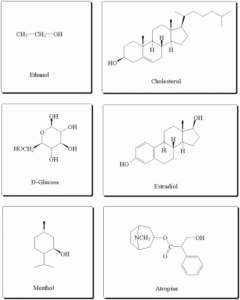
1. Alcohols
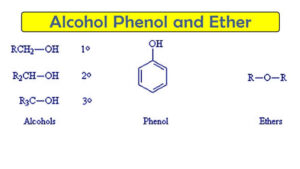
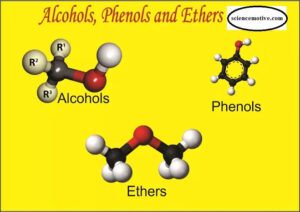
Alcohols are compounds where a hydroxyl group (-OH) is attached to a saturated carbon atom (a carbon that is only bonded by single bonds).
General Formula: CnH₂n+₁OH
Examples:
Methanol (CH₃OH) – used as a fuel and solvent
Ethanol (C₂H₅OH) – the type of alcohol found in alcoholic drinks
Propanol (C₃H₇OH) – used in disinfectants
Types of Alcohols (based on structure):
Primary (1°): –OH is on a carbon attached to one other carbon
Secondary (2°): –OH is on a carbon attached to two other carbons
Tertiary (3°): –OH is on a carbon attached to three other carbons
2. Phenols
Phenols are aromatic compounds where the -OH group is directly attached to a benzene ring.
Example:
Phenol (C₆H₅OH) – used in antiseptics and disinfectants
Phenols are more acidic than alcohols because the benzene ring stabilises the negative charge formed when the hydrogen atom from -OH is lost.
3. Ethers
Ethers are compounds where an oxygen atom is bonded to two alkyl or aryl groups.
General Formula: R-O-R′
Examples:
Diethyl ether (CH₃CH₂-O-CH₂CH₃) – was used as an anaesthetic
Methyl tert-butyl ether (MTBE) – used in petrol as an additive
Ethers are generally less reactive than alcohols and phenols but are useful solvents in organic reactions.
Physical Properties
Alcohols and phenols can form hydrogen bonds, so they tend to have higher boiling points.
Ethers cannot hydrogen bond with themselves (no -OH), so they boil at lower temperatures than alcohols of similar size.
Solubility in water decreases as the carbon chain gets longer, but small alcohols and phenols dissolve well.
Common Uses in Daily Life
Alcohols: hand sanitisers, fuels, cosmetics, pharmaceuticals
Phenols: antiseptics, production of plastics like Bakelite
Ethers: solvents, fuel additives, perfumes
Summary
- Alcohols contain -OH on a saturated carbon; classified as primary, secondary, or tertiary.
- Phenols have -OH attached directly to a benzene ring and are more acidic.
- Ethers have oxygen linking two carbon groups and are used mainly as solvents.
- Their physical and chemical properties depend on how the oxygen is bonded.
Evaluation
- What is the structural difference between alcohols and phenols?
- Explain why phenols are more acidic than alcohols.
- Give two everyday uses of ethers.
- Classify the alcohol in the compound CH₃CH(OH)CH₃.
Fantastic work today! These compounds may seem similar, but now you can clearly tell them apart and even recognise them around you. Your chemistry knowledge is growing stronger every day. Keep showing up with this great energy — Afrilearn is proud to be part of your learning journey. See you in the next lesson!
