Back to: Organic Chemistry 200 Level
Welcome to class!
Hello brilliant learner! I’m so glad you’re here today. We’re going to start talking about two important families of organic compounds — Aldehydes and Ketones. You might not know it, but these compounds are all around you: from the sweet smell of vanilla flavouring to the chemicals used in preserving food and making perfumes. By the end of today’s lesson, you’ll be able to recognise them, name them, and understand why they behave the way they do.
Aldehydes And Ketones I
What Are Aldehydes and Ketones?
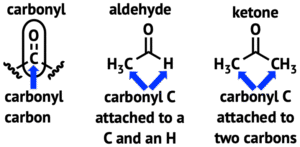
Both aldehydes and ketones belong to a group of organic compounds called carbonyl compounds. This is because they both contain the carbonyl group (C=O) — a carbon atom double-bonded to oxygen.
Key Difference:
Aldehydes: The carbonyl group is at the end of the carbon chain. At least one hydrogen atom is directly attached to the carbonyl carbon.
Ketones: The carbonyl group is in the middle of the carbon chain, with two carbon atoms attached to the carbonyl carbon.
General Formulas:
Aldehydes: R–CHO
Ketones: R–CO–R′
Examples:
Aldehydes: Methanal (formaldehyde), Ethanal (acetaldehyde)
Ketones: Propanone (acetone), Butanone
Naming Aldehydes and Ketones (IUPAC Rules)
Identify the longest carbon chain containing the carbonyl group.
For aldehydes, change the -e ending of the alkane name to -al (e.g., ethane → ethanal).

For ketones, change the -e to -one and indicate the position of the carbonyl group if necessary (e.g., pentan-2-one).
Number the carbon atoms so that the carbonyl carbon gets the lowest possible number.
Physical Properties
Boiling points: Higher than alkanes of similar size (because of polar C=O bond) but lower than alcohols (because they cannot form hydrogen bonds with themselves).
Solubility: Smaller aldehydes and ketones dissolve well in water because they can form hydrogen bonds with water molecules. As the carbon chain gets longer, solubility decreases.
Uses in Everyday Life
Methanal: Used to preserve biological specimens.
Ethanol: Used in flavourings, perfumes, and as an intermediate in manufacturing.
Propanone (Acetone): Commonly used as a nail polish remover and solvent.
Summary
- Aldehydes and ketones contain the carbonyl group (C=O).
- Aldehydes have the carbonyl group at the end of the chain; ketones have it in the middle.
- They are named by replacing -e with -al (aldehydes) or -one (ketones).
- They have higher boiling points than hydrocarbons but lower than alcohols, and small ones are soluble in water.
Evaluation
- What functional group is common to both aldehydes and ketones?
- How do you distinguish between the position of the carbonyl group in aldehydes and ketones?
- Name CH₃CH₂CH₂CHO using IUPAC rules.
- Give one use of propanone.
Excellent work today! You’ve just taken your first confident steps into understanding aldehydes and ketones — two important classes of compounds with real-life applications all around you. Keep this curiosity alive, and remember that with Afrilearn, you’re building a strong foundation for mastery in Chemistry. See you in the next lesson!
