Back to: Organic Chemistry 200 Level
Welcome to class!
Hi brilliant scholar! It’s wonderful to see you back for another exciting lesson. Yesterday we got to know aldehydes and ketones — what they are, how to name them, and some of their properties. Today, we’ll go deeper into their chemical reactions and learn how they behave when they meet other chemicals. These reactions are the reason aldehydes and ketones are so useful in medicine, industry, and everyday products.
Aldehydes And Ketones II
General Reactivity of Aldehydes and Ketones
The main reactive site in both aldehydes and ketones is the carbonyl group (C=O). The carbon atom in C=O is electrophilic — meaning it loves to accept electrons — because oxygen pulls electron density away from it. This makes it attractive to nucleophiles (electron-rich species).
Oxidation Reactions
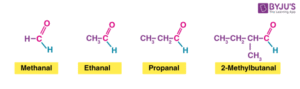
Aldehydes: Easily oxidised to carboxylic acids. This is because they have a hydrogen atom attached to the carbonyl carbon.
Example: Ethanal + [O] → Ethanoic acid.
Common oxidising agents: Acidified potassium dichromate (K₂Cr₂O₇/H₂SO₄), Tollens’ reagent, Fehling’s solution.
Ketones: Resistant to oxidation under mild conditions. They require strong oxidising agents, and oxidation usually breaks the carbon chain.
Reduction Reactions
Both aldehydes and ketones can be reduced to alcohols:
Aldehydes → Primary alcohols.
Ketones → Secondary alcohols.
Common reducing agents: Lithium aluminium hydride (LiAlH₄) or sodium borohydride (NaBH₄).
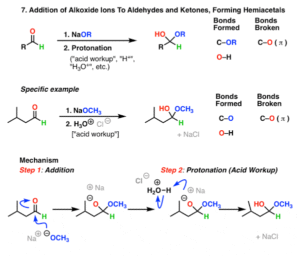
Nucleophilic Addition Reactions
This is the most important reaction type for aldehydes and ketones.
The nucleophile attacks the carbonyl carbon, breaking the double bond.
Hydrogen cyanide (HCN) addition forms cyanohydrins, useful intermediates in organic synthesis.
Tests to Distinguish Aldehydes from Ketones
Tollens’ Test: Aldehydes give a silver mirror; ketones do not.
Fehling’s Test: Aldehydes give a brick-red precipitate; ketones do not.
These are possible because aldehydes can be oxidised easily, but ketones cannot.
Everyday Relevance
Aldehyde reactions help produce perfumes, flavourings, and pharmaceuticals.
Ketone stability makes them useful as solvents for paints and plastics.
Diagnostic tests like the silver mirror test are applied in labs to confirm the presence of aldehydes in compounds.
Summary
- Aldehydes oxidise easily to carboxylic acids; ketones resist mild oxidation.
- Both can be reduced to alcohols using LiAlH₄ or NaBH₄.
- Nucleophilic addition is the key reaction type for both.
- Aldehydes can be distinguished from ketones using Tollens’ or Fehling’s test.
Evaluation
- Why are aldehydes more easily oxidised than ketones?
- What products are formed when aldehydes and ketones are reduced?
- Name one reagent used to distinguish between aldehydes and ketones.
- Write the equation for the oxidation of propanal to propanoic acid.
Well done! You’ve just mastered the major reactions of aldehydes and ketones — knowledge that will be essential in your journey through Organic Chemistry. Keep your mind sharp and your curiosity alive. With Afrilearn, you’re not just learning Chemistry — you’re preparing to solve real-world problems with science. See you in the next lesson!
